In-situ reactive oxygen species and myeloperoxidase responsive self-luminous polymer material as well as preparation method and application thereof
A myeloperoxidase and polymer material technology, applied in the field of biomedicine, can solve the problems of short wavelength, limited in vivo imaging applications, poor tissue penetration of light waves, etc. The effect of diversification of drug routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
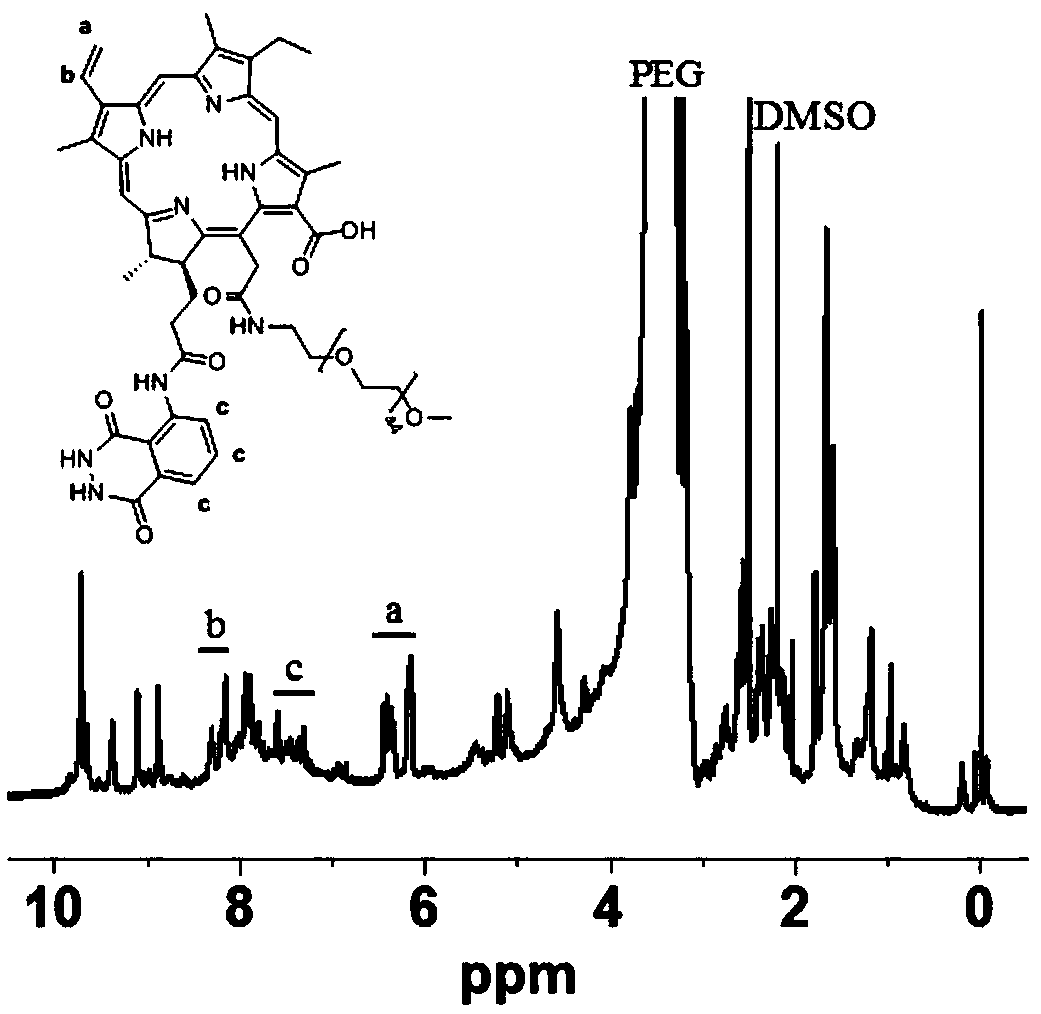
[0042]Under nitrogen protection, 0.1mM chlorin e6 and 1mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride / N-hydroxysuccinimide were dissolved in 5ml anhydrous dihydrogen In methyl sulfoxide, react at 0°C for 12 hours to obtain the chlorin e6 intermediate with activated carboxyl group; add 0.2mM luminol (corresponding to the content of the invention, the first substituent in ①), and react at 0°C for 24 hours to obtain Luminol-substituted chlorin e6 compound; then, add 0.2mM molecular weight of 400Da amino-terminated polyethylene glycol, react at 0°C for 24 hours to obtain chlorin e6 bonded luminol as hydrophobic unit, polyethylene glycol as the amphiphilic polymer of the hydrophilic segment; finally, the amphiphilic polymer is transferred to a dialysis bag with a molecular weight cut-off of 1000Da, dialyzed in ultrapure water for 48 hours, and freeze-dried at -60°C to obtain Reactive oxygen and myeloperoxidase responsive self-luminescent polymer materials.
Embodiment 2
[0044] Under nitrogen protection, 0.1mM chlorin e6 and 1.5mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride / N-hydroxysuccinimide in 10ml without In water N,N-dimethylformamide, react at 25°C for 18 hours to obtain the carboxyl-activated chlorin e6 intermediate; add 1mM isoluminol (corresponding to the content of the invention, the second substituent in ①), 25 ℃ reaction for 72 hours to obtain isoluminol-substituted chlorin e6 compound; add 0.5mM amino-terminated polyethylene glycol with a molecular weight of 1000Da, and react at 25 ℃ for 72 hours to obtain chlorin e6-isoluminol The amphiphilic polymer is a hydrophobic unit and polyethylene glycol is a hydrophilic segment; the amphiphilic polymer is transferred to a dialysis bag with a molecular cutoff of 2000, dialyzed in ultrapure water for 72 hours, and freeze-dried at -57°C. Reactive oxygen and myeloperoxidase-responsive self-luminescent polymer materials were obtained.
Embodiment 3
[0046] Under nitrogen protection, 0.1mM chlorin e6 and 2mM 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride / N-hydroxysuccinimide in 20ml of anhydrous In N,N-dimethylacetamide, react at 50°C for 24 hours to obtain the carboxyl-activated chlorin e6 intermediate; add 2mM 8-amino-5-chloro-7-phenylpyrido[3,4-di] Pyridazine-1,4(2H,3H)dione (L-012) (corresponding to the content of the invention, the third substituent in ①), react at 50°C for 100 hours to obtain 8-amino-5-chloro-7-phenyl Pyrido[3,4-di]pyridazine-1,4(2H,3H)dione substituted chlorin e6 compound; add 2mM amino-terminated polyethylene glycol with a molecular weight of 2000, and react at 50°C for 100 hours An amphiphilic polymer with chlorin e6 / L-012 as the hydrophobic unit and polyethylene glycol as the hydrophilic segment was obtained; the amphiphilic polymer was transferred to a dialysis bag with a molecular cutoff of 5000 Da, and ultrapure water Dialyzed in medium for 72 hours, and freeze-dried at -55°C to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com