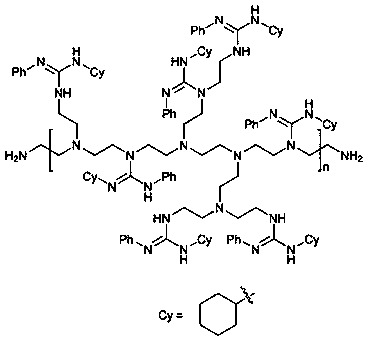
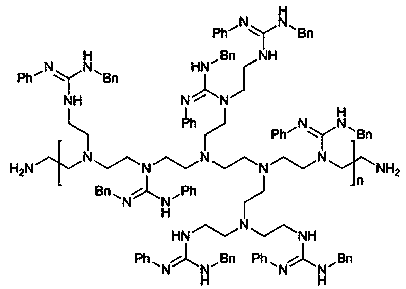
Preparation method of guanidine polymer heterogeneous catalyst as well as application method of guanidine polymer heterogeneous catalyst to catalytic synthesis of Warfarin and derivatives thereof
A technology of a heterogeneous catalyst and an application method, applied in the application of catalytic synthesis of warfarin and its derivatives, the field of preparation of a guanidine polymer heterogeneous catalyst, can solve the problems of increasing the reaction cost and operation steps, and achieve mild conditions. , The effect of simple purification and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of Diphenyl-Substituted Guanidine Polymers
[0030] Take 1.86 g of aniline and 2.71 g of phenyl isothiocyanate dissolved in 50 mL of acetonitrile, stir at room temperature for 6 hours, a large amount of white precipitate appears, filter and dry to obtain 4.22 g of white solid, yield 92.5%.
[0031] Take 2.28 g of N,N'-diphenylthiourea obtained in the previous step, dissolve it in 50 mL of dichloromethane, then add 3.03 g of triethylamine, and finally add 5.11 g of 2-chloro-1-methyliodopyridine, Stir at room temperature for 30 minutes, filter, spin the filtrate to remove the solvent, and separate and purify by column chromatography (eluent: petroleum ether) to obtain 1.57 g of a colorless transparent liquid with a yield of 81.0%.
[0032] 1.57 g (about 8 mmol) of diphenylcarbodiimide obtained in the previous step was dissolved in 20 mL of dichloromethane, and 0.473 g of branched polyethyleneimine (1 mmol equivalent, molecular weight 10,000) was added...
Embodiment 2
[0034] Embodiment 2: the synthesis of phenyl, benzyl substituted guanidine polymer
[0035] Take 2.14 g of benzylamine and 2.71 g of phenyl isothiocyanate dissolved in 50 mL of acetonitrile, stir at room temperature for 6 hours, a large amount of white precipitate appears, filter and dry to obtain 4.56 g of white solid, yield 94.0%.
[0036] Take 2.42 g of thiourea obtained in the previous step, dissolve it in 50 mL of dichloromethane, then add 3.03 g of triethylamine, and finally add 5.11 g of 2-chloro-1-methyliodopyridine, stir at room temperature for 30 minutes, and filter. After the filtrate was spun to remove the solvent, it was separated and purified by column chromatography (eluent: petroleum ether) to obtain 1.66 g of a colorless transparent liquid with a yield of 79.8%.
[0037] 1.66 g (about 7.9 mmol) of the carbodiimide obtained in the previous step was dissolved in 20 mL of dichloromethane, and 0.473 g of branched polyethyleneimine (1 mmol equivalent, molecular wei...
Embodiment 3
[0039] Embodiment 3: the synthesis of phenyl, n-hexyl substituted guanidine polymer
[0040] 2.02 g of n-hexylamine and 2.71 g of phenylisothiocyanate were dissolved in 50 mL of acetonitrile, and stirred at room temperature for 6 hours, a large amount of white precipitate appeared, which was filtered and dried to obtain 4.12 g of white solid with a yield of 87.1%.
[0041] Take 2.36 g of thiourea obtained in the previous step, dissolve it in 50 mL of dichloromethane, then add 3.03 g of triethylamine, and finally add 5.11 g of 2-chloro-1-methyliodopyridine, stir at room temperature for 30 minutes, and filter. After the filtrate was spun to remove the solvent, it was separated and purified by column chromatography (eluent: petroleum ether) to obtain 1.26 g of a colorless transparent liquid with a yield of 62.3%.
[0042] 1.26 g (about 6.23 mmol) of the carbodiimide obtained in the previous step was dissolved in 20 mL of dichloromethane, and 0.368 g of branched polyethyleneimine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com