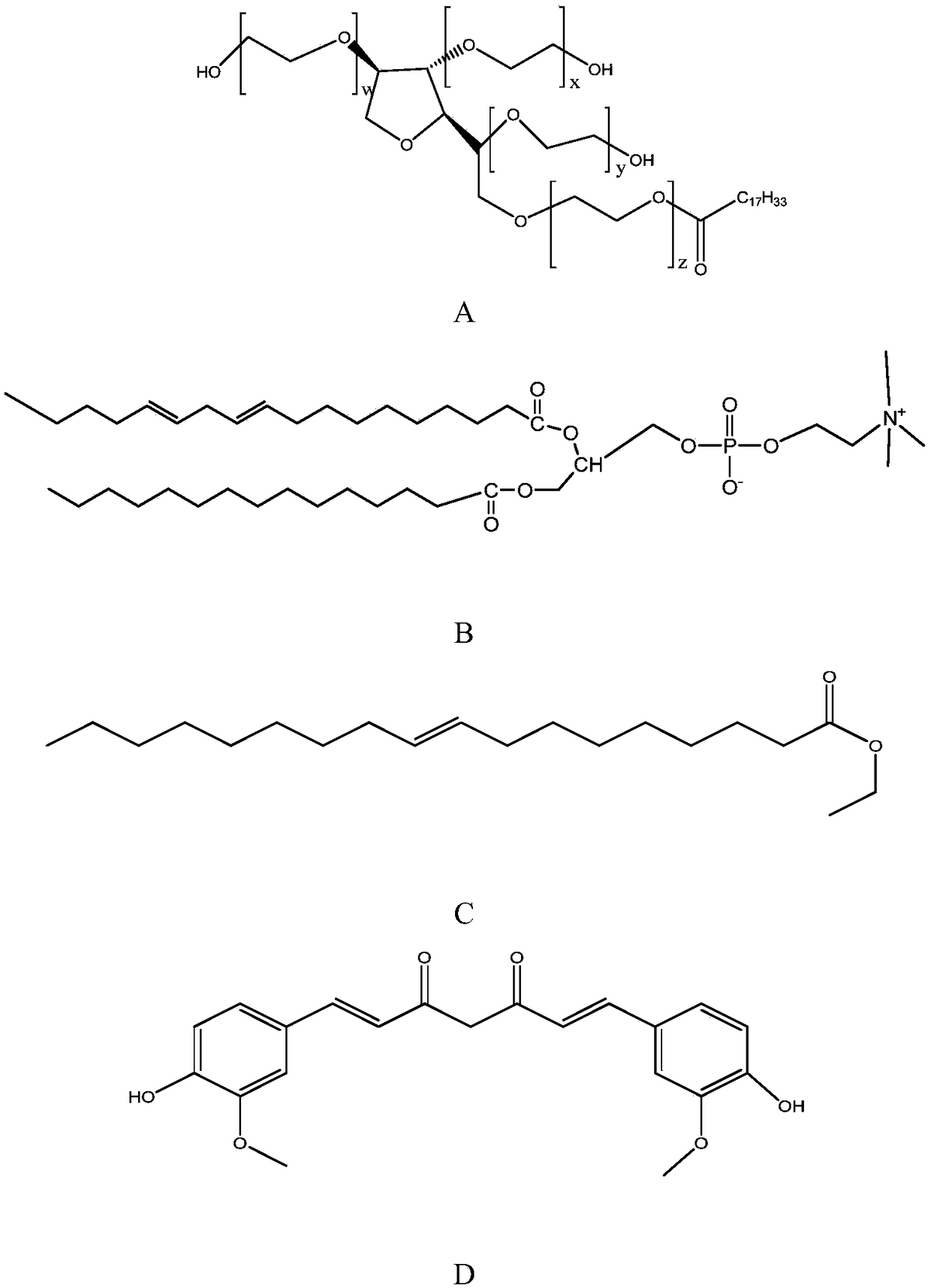
A lyotropic liquid crystal drug carrier and a preparation method and application thereof
A technology of lyotropic liquid crystal and ethyl oleate, which is used in drug combinations, pharmaceutical formulations, antipyretics and other directions to achieve the effects of improving drug release properties, improving drug solubility and stability, and good sustained release effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
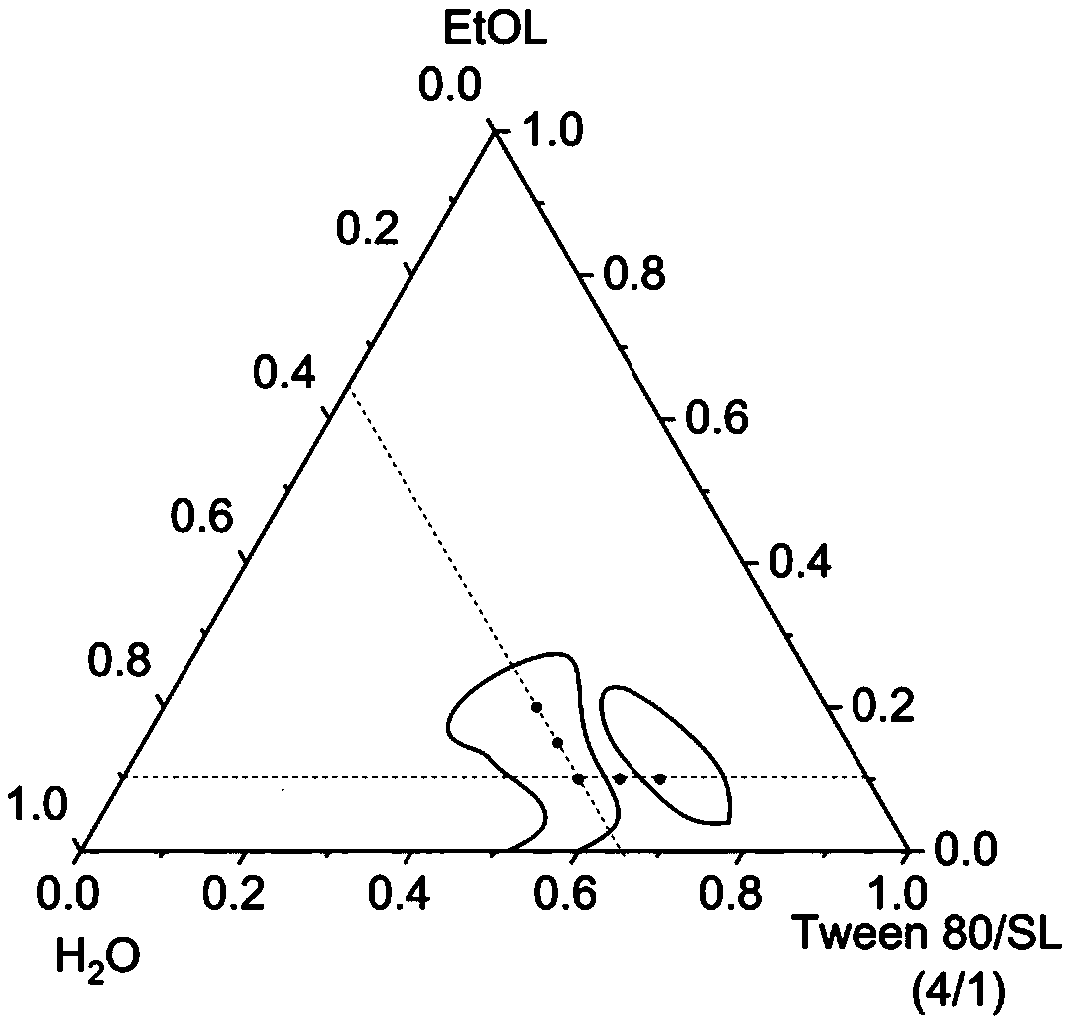
[0053] 1. Drawing of phase diagram
[0054] First, according to the molar ratio of 4 / 1, accurately weigh the surfactant and place it in a colorimetric tube, and mix well; secondly, according to the change of the ratio of the surfactant to the oil phase from 10:0 to 0:10, accurately weigh the oleic acid in sequence Ethyl ester in different colorimetric tubes, stir and mix well; finally, add double distilled water drop by drop to the colorimetric tubes, the percentage of water increases at intervals of 2%, use magnetic stirring in a water bath at 40-50°C Stir evenly with a mixer, then place in a water bath at 25°C to balance, observe and record the changes in the phase state and appearance of the aggregates, and it is necessary to extend the equilibrium time of the aggregates when approaching the phase boundary. The phase boundary is preliminarily judged by visually observing the color, transparency, hardness, viscosity, etc. of the aggregates.
[0055] 2. Preparation of sample...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com