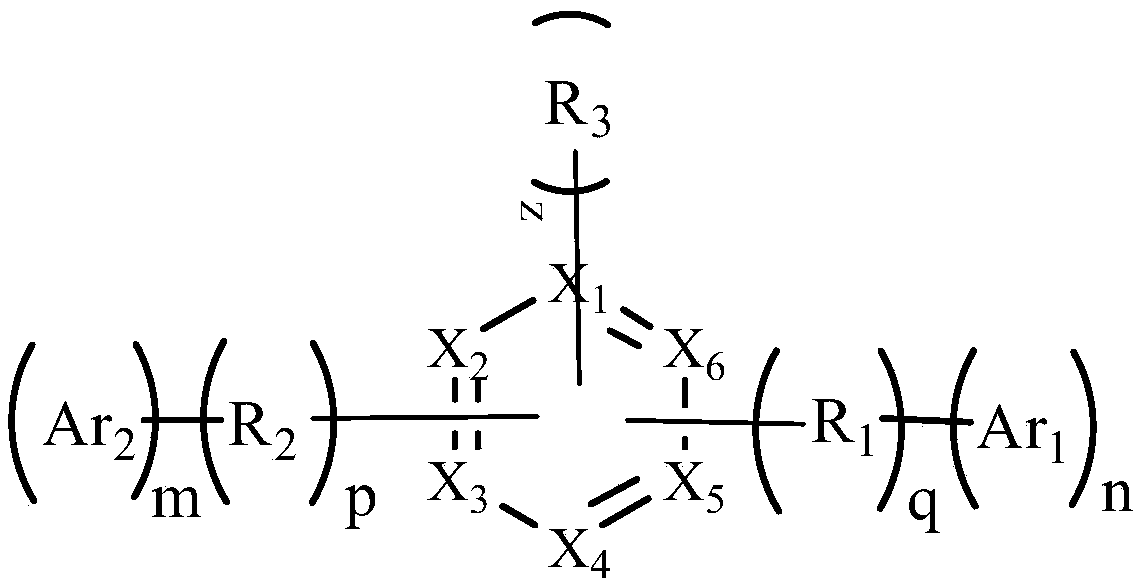
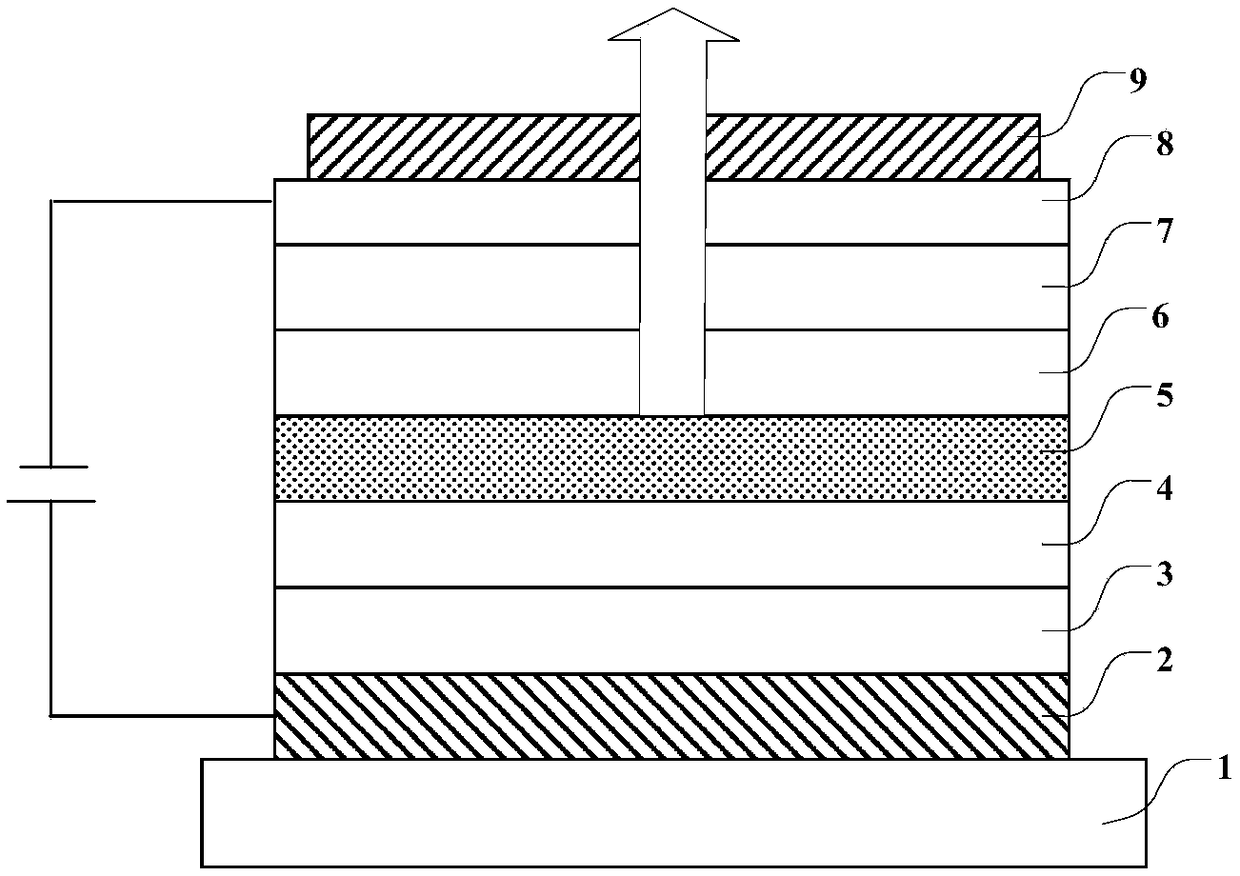
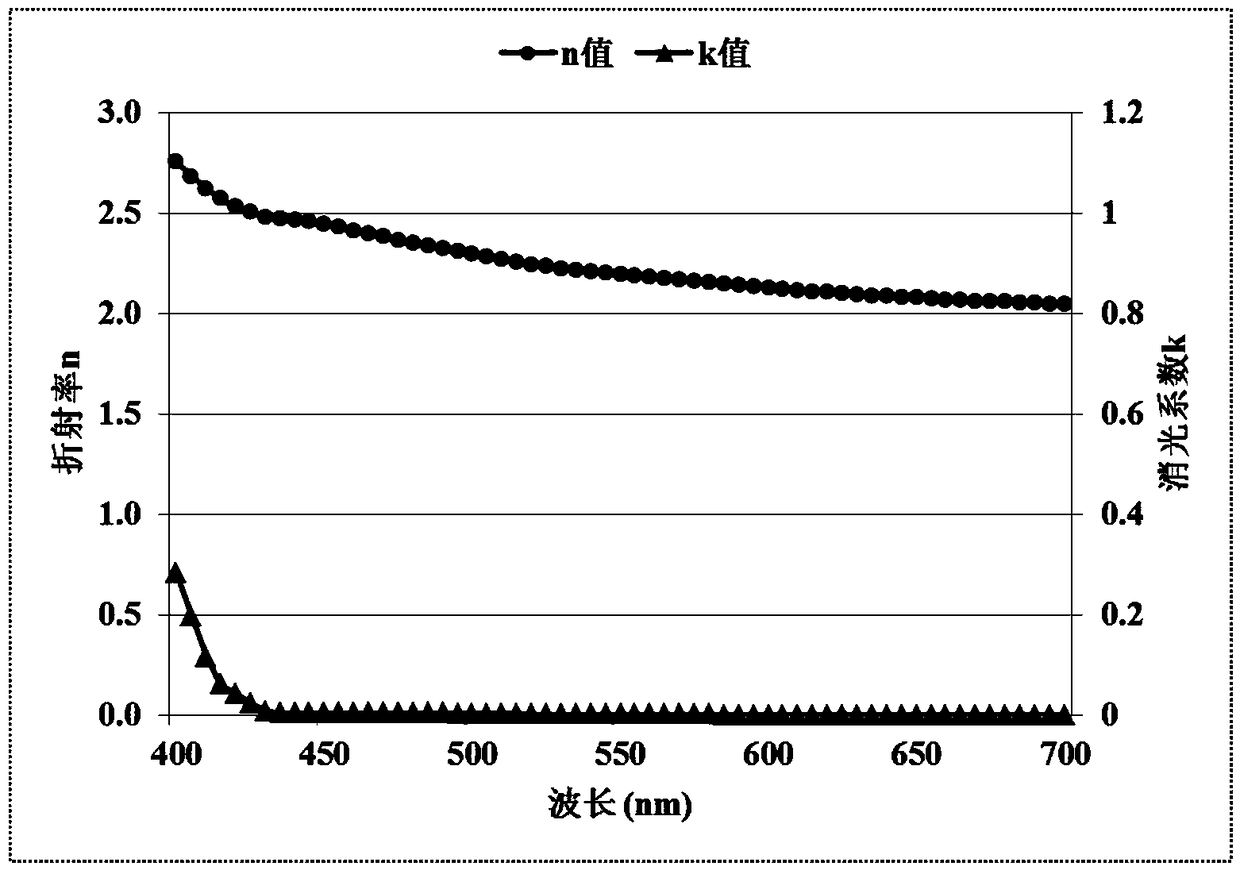
Nitrogen heterocyclic, display panel, and display device
A nitrogen heterocyclic compound and aromatic heterocyclic technology is applied in the field of organic electroluminescent materials, which can solve the problems that the molecules cannot be tightly packed, cannot meet the high refractive index, and cannot solve the luminous efficiency, etc., and achieves short conjugation length, The effect of improving luminous efficiency and improving light extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Synthesis of compound CP2
[0075]
[0076] In a 250ml three-necked flask, under nitrogen protection, in 150ml DMF, successively add raw material intermediate M1 (0.012mol), 3-phenanthrolinyl borate (0.025mol) and palladium acetate (0.0003mol), mix and stir, and then Join K 3 PO 4 (0.045mol) aqueous solution, reflux reaction at a temperature of 130°C for 10h. Naturally cool to room temperature, after the reaction, add 100mL deionized water, then drop a few drops of 2M HCl, extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography, and finally purified to obtain the product CP2.
[0077] Compound CP2 elemental analysis result (molecular formula C 40 h 24 N 6 ): theoretical value: C, 81.61; H, 4.11; N, 14.28. Test values: C, 81.63; H, 4...
Embodiment 2
[0079] Synthesis of compound CP4:
[0080]
[0081] In a 250ml three-necked flask, under nitrogen protection, in 150ml DMF, successively add raw material intermediate M1 (0.012mol), 4-quinolyl borate (0.025mol) and palladium acetate (0.0003mol), mix and stir, Then join K 3 PO 4 (0.045mol) aqueous solution, reflux reaction at a temperature of 130°C for 10h. Naturally cool to room temperature, after the reaction, add 100mL deionized water, then drop a few drops of 2M HCl, extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography, and finally purified to obtain the product CP4.
[0082] Compound CP4 element analysis result (molecular formula C 34 h 22 N 4 ): Theoretical values: C, 83.93; H, 4.56; N, 11.51. Test values: C, 83.95; H, 4.54; N, 11.51...
Embodiment 3
[0084] Synthesis of compound CP8:
[0085]
[0086] In a 250ml three-necked flask, under nitrogen protection, in 150ml DMF, successively add raw material intermediate M1 (0.012mol), 4-phenanthrolinyl borate (0.025mol) and palladium acetate (0.0003mol), mix and stir, and then Join K 3 PO 4 (0.045mol) aqueous solution, reflux reaction at a temperature of 130°C for 10h. Naturally cool to room temperature, after the reaction, add 100mL deionized water, then drop a few drops of 2M HCl, extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel chromatography, and finally the product CP8 was obtained by purification.
[0087] Compound CP8 (molecular formula C 40 h 24 N 6 ) Elemental analysis results: theoretical value: C, 81.61; H, 4.11; N, 14.28. Test values: C, 81.64;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com