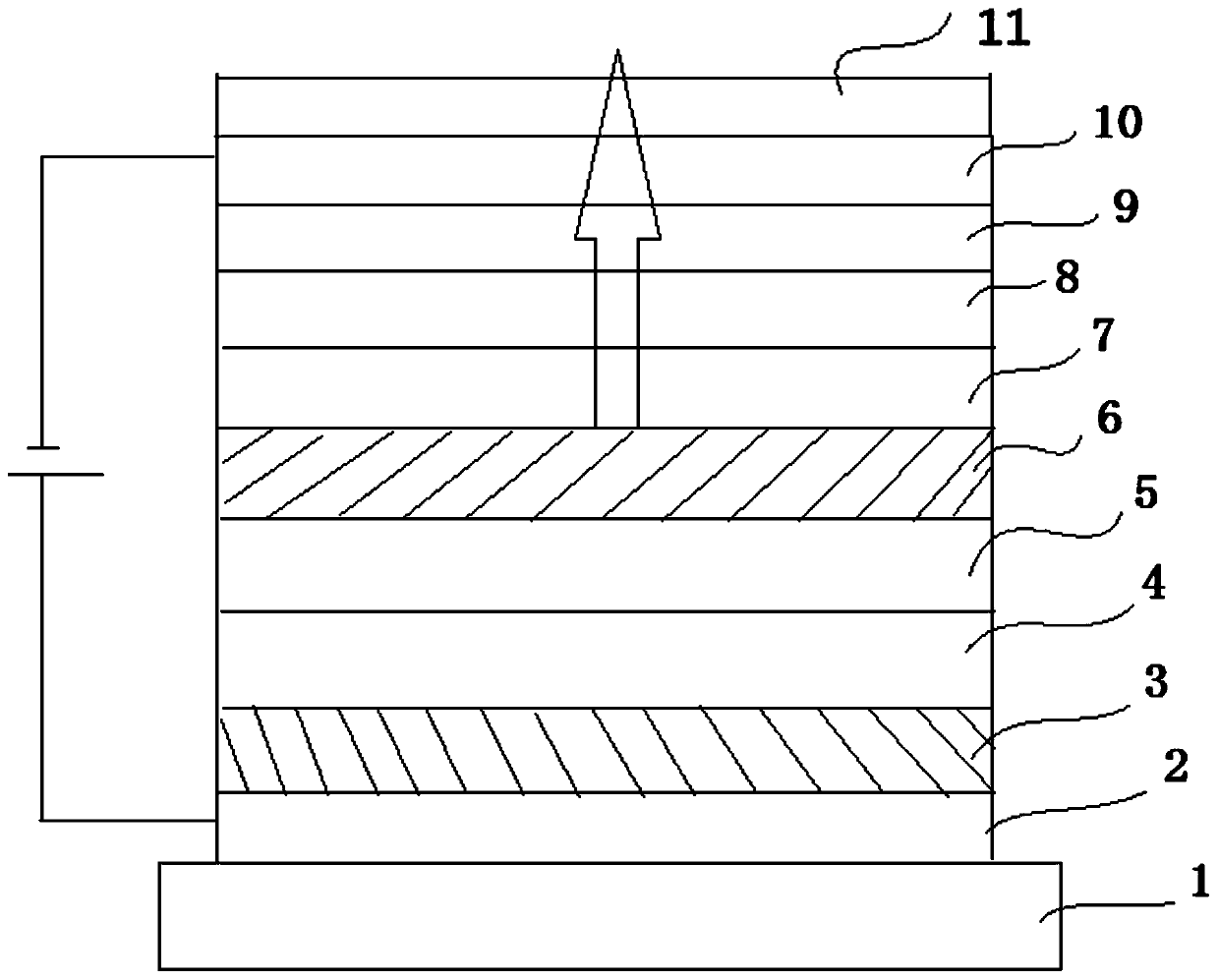
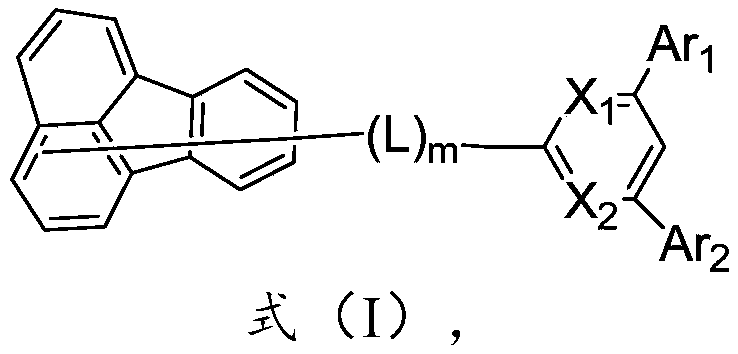
Compound, display panel and display device
A compound and connection position technology, applied in the field of organic electroluminescent materials, can solve problems such as energy loss, achieve small extinction coefficient, improve light extraction efficiency, and improve luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145]
[0146](1) In a 250ml round bottom flask, compound 2-1 (15mmol), diethyl malonate (35mmol) and sodium ethoxide (15mmol) were added to dry ethanol (100ml), under nitrogen atmosphere, temperature React at 78°C for 12 hours, add the obtained intermediate mixed solution into water, then filter through a pad of diatomaceous earth, extract the filtrate with dichloromethane, then wash with water, and dry with anhydrous magnesium sulfate, filter and evaporate , the crude product was purified by silica gel column chromatography to obtain the intermediate compound 3-1.
[0147] (2) In a 250ml round bottom flask, add compound 3-1 (15mmol), diethylphenylamine (15mol) to dry POCl 3 (100ml), under nitrogen atmosphere, the temperature is 6.0 hours under the condition of 120 ℃, and the obtained intermediate mixed solution is added in water, then filtered through a diatomaceous earth pad, and the filtrate is extracted with dichloromethane, then washed with water, and After drying o...
Embodiment 2
[0153]
[0154] In a 250ml round bottom flask, 9-anthraceneboronic acid pinacol ester (20mmol), compound 5-1 (10mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to a mixture of toluene (30ml) / ethanol (20ml) and potassium carbonate (12mmol) aqueous solution (10ml), and the reaction was refluxed under nitrogen atmosphere for 12h. The resulting mixture was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude The product yielded the final product Compound P3.
[0155] 1 H NMR (400MHz, CDCl 3 )δ8.27 (s, 2H), 7.91-7.58 (m, 21H), 7.54 (d, J = 8.4Hz, 4H), 7.28 (s, 1H), 7.26-7.24 (m, 4H);
[0156] Characterization results: elemental analysis results of compound P3 (molecular formula C 54 h 32 N 2 ): theoretical value: C, 91.53; H, 4.52; N, 3.95. Test values: C, 91.53; H, 4.52; N, 3.95. ESI-MS (m / z)...
Embodiment 3
[0158]
[0159] In a 250ml round bottom flask, 9-phenanthrene borate pinacol ester (20mmol), compound 5-1 (10mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to a mixture of toluene (30ml) / ethanol (20ml) and potassium carbonate (12mmol) aqueous solution (10ml), and the reaction was refluxed under nitrogen atmosphere for 12h. The resulting mixture was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude The product yielded the final product Compound P8.
[0160] 1 H NMR (400MHz, CDCl 3 )δ8.93-7.82 (m, 16H), 7.93 (s, 2H), 7.91-7.58 (m, 5H), 7.54 (d, J = 8.4Hz, 4H), 7.28 (s, 1H), 7.26-7.24 (m,4H);
[0161] Characterization results: elemental analysis results of compound P8 (molecular formula C 54 h 32 N 2 ): theoretical value: C, 91.53; H, 4.52; N, 3.95. Test values: C, 91.53; H, 4.52; N, 3.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com