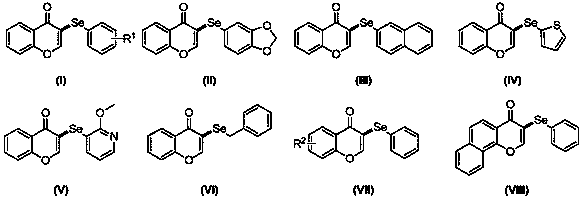
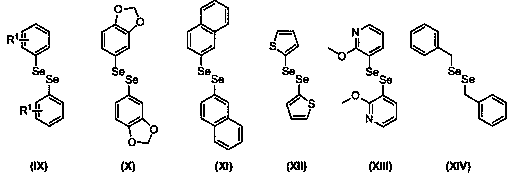
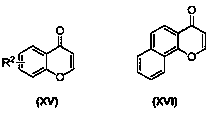
Preparation method of seleno-flavonoid compounds
A technology of chromone compounds and selenoflavones, which is applied in organic chemistry and other fields, can solve the problems of narrow substrate range and achieve the effects of short reaction time, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add chromone (0.2mmol), 1,2-diphenyldiselenide (0.1 mmol), NIS (0.04mmol), TBHP (0.8mmol) and DMF (2.0mL) into a 5mL reaction flask respectively, and stir at 70°C. TLC tracking detection reaction. After 7 hours, the reaction was stopped. Water and ethyl acetate were added to the reaction system, and the organic layer was separated. The aqueous layer was washed twice with ethyl acetate, all organic layers were combined and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (10% ethyl acetate petroleum ether solution) to obtain 56.6 mg of the product (CAS No.: 1980869-97-3), with a yield of 94%. The process is as follows:
[0028]
[0029] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0030] 1 H NMR (500MHz, DMSO-d 6 )δ8.61(s,1H),8.07(dd,J=8.0,1.4Hz,1H),7.85(dd,J=11.3,4.3Hz,1H),7.68(dd,J=8.5,0.6Hz,1H ),7.55–7.52 (m,1H),7.47–7.4...
Embodiment 2
[0032] Add chromone (0.2mmol), 1,2-diphenyldiselenide (0.1 mmol), NIS (0.04mmol), TBHP (0.8mmol) and DMSO (2.0mL) into a 5mL reaction flask respectively, and stir at 70°C. TLC tracking detection reaction. After 12 hours, the reaction was stopped. Water and ethyl acetate were added to the reaction system, and the organic layer was separated. The aqueous layer was washed twice with ethyl acetate, all organic layers were combined and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (10% ethyl acetate petroleum ether solution) to obtain 27.1 mg of product with a yield of 45%. The reaction process is shown in the following formula:
[0033]
[0034] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0035] 1 H NMR (500MHz, DMSO-d 6 )δ8.61(s,1H),8.07(dd,J=8.0,1.4Hz,1H),7.85(dd,J=11.3,4.3Hz,1H),7.68(dd,J=8.5,0.6Hz,1H ),7.55–7.52 (m,1H),7.47–7....
Embodiment 3
[0037] Add chromone (0.2mmol), 1,2-diphenyldiselenide (0.1 mmol), NIS (0.04mmol), TBHP (0.8mmol) and DCM (2.0mL) into a 5mL reaction flask respectively, and stir at 70°C. TLC tracking detection reaction. After 12 hours, the reaction was stopped. Water and ethyl acetate were added to the reaction system, and the organic layer was separated. The aqueous layer was washed twice with ethyl acetate, all organic layers were combined and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (10% ethyl acetate petroleum ether solution) to obtain 12.6 mg of product with a yield of 21%. The reaction process is shown in the following formula:
[0038]
[0039]Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0040] 1 H NMR (500MHz, DMSO-d 6 )δ8.61(s,1H),8.07(dd,J=8.0,1.4Hz,1H),7.85(dd,J=11.3,4.3Hz,1H),7.68(dd,J=8.5,0.6Hz,1H ),7.55–7.52 (m,1H),7.47–7.44...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com