Cation free radical type covalent organic frame material as well as preparation method and application thereof
A covalent organic framework and free radical technology, applied in the field of functional materials, can solve the problems of complex preparation process, low photothermal conversion rate, and difficulty in meeting the actual needs of photothermal therapy, and achieve simple operation, high specific surface area, and process controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
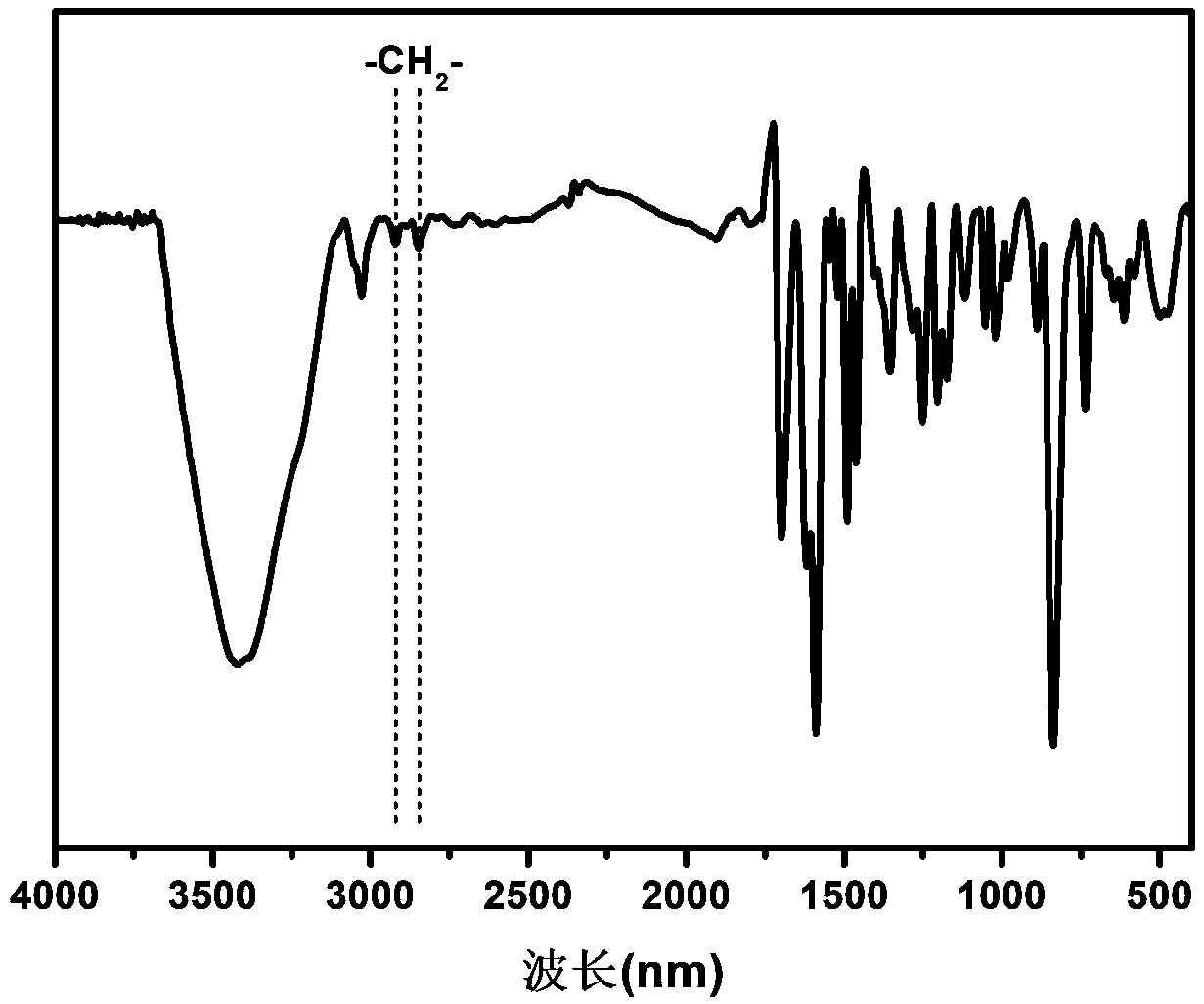
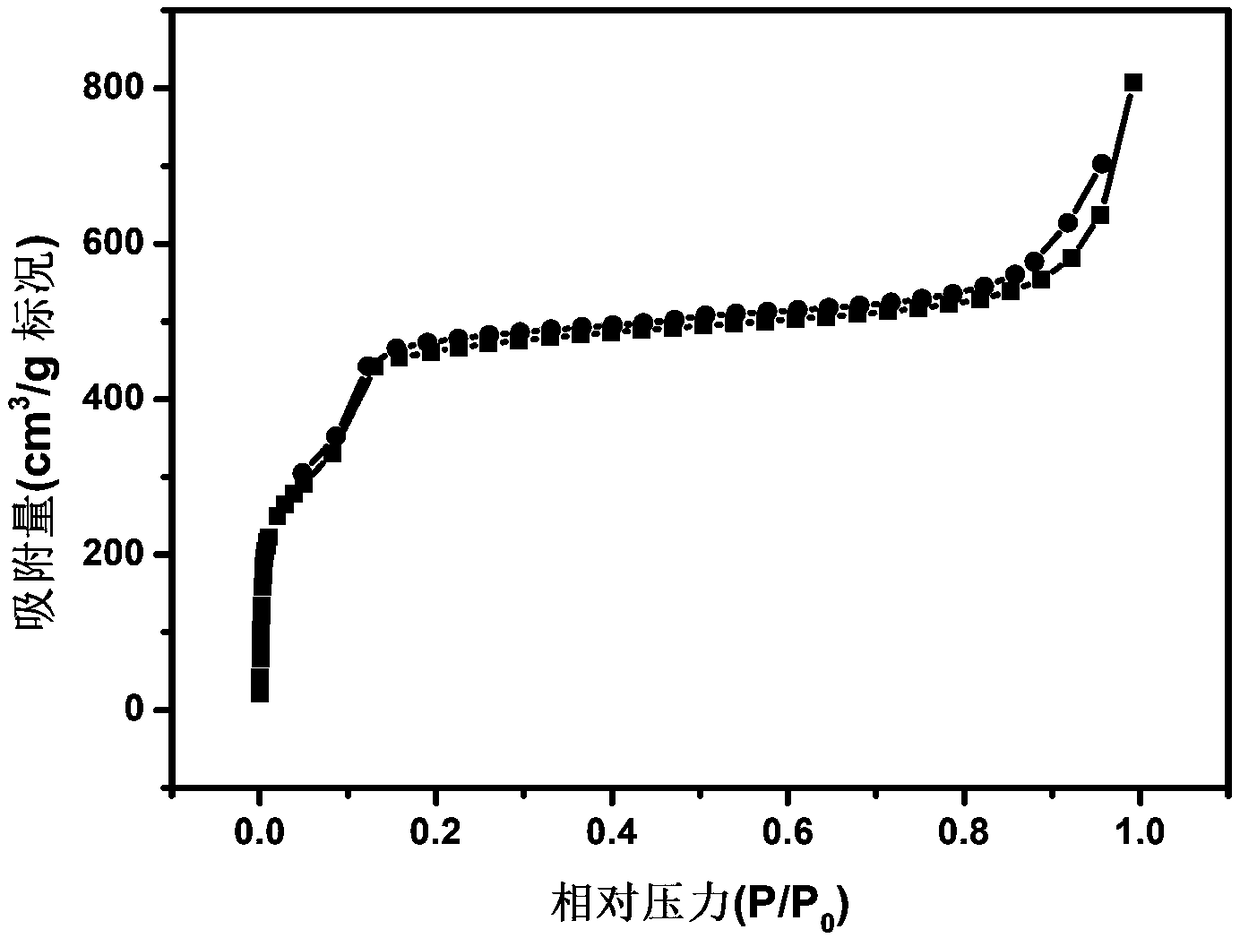
Image
Examples
Embodiment 1
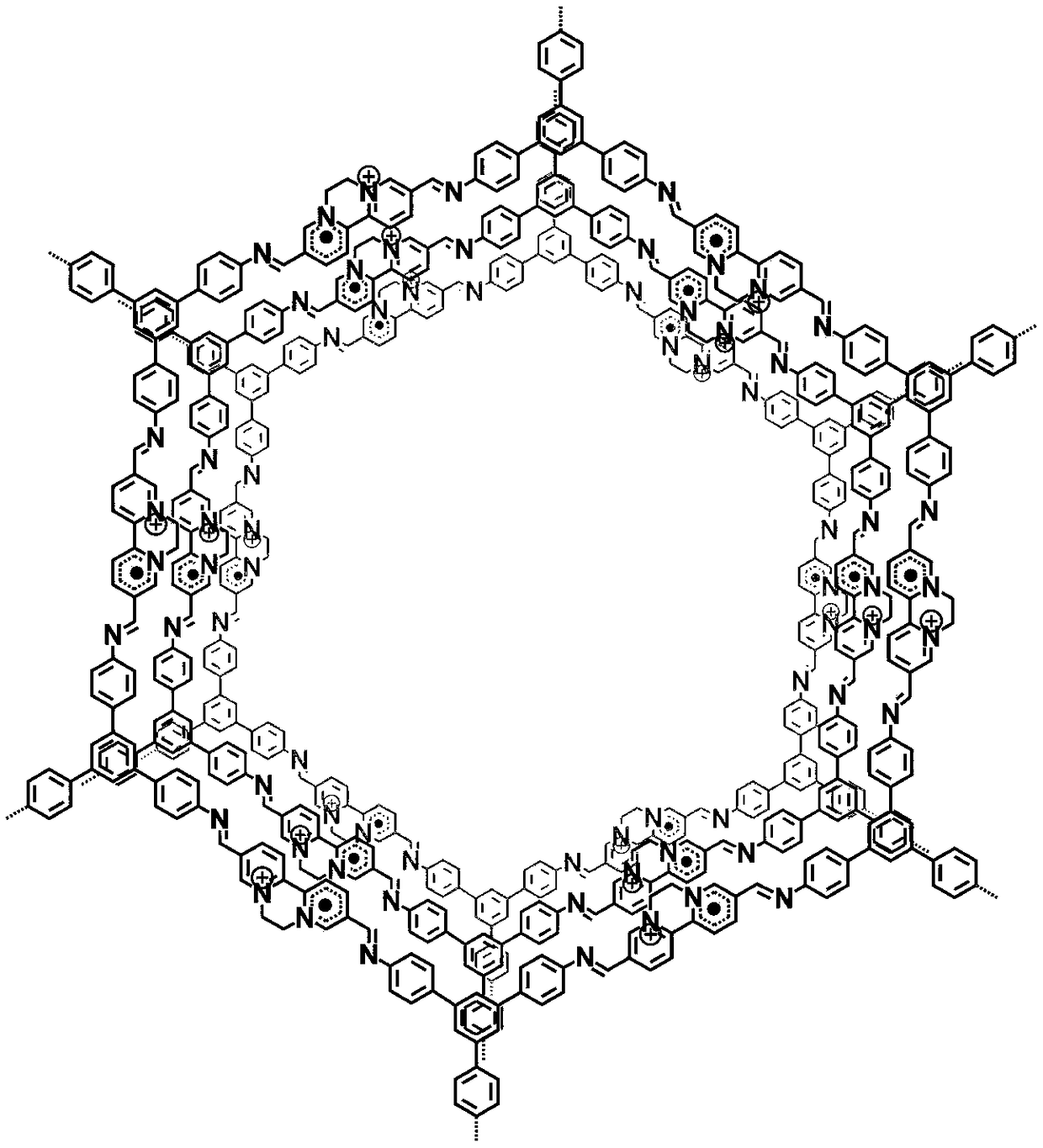
[0037] Example 1 relates to a cationic free radical type covalent organic framework material, its preparation method and its near-infrared photoresponse photothermal conversion application.
[0038] The preparation method of the cationic radical type covalent organic framework material in Example 1 is as follows:
[0039] Step 1, preparation of covalent organic framework materials linked by imine bonds containing nitrogen heterocycles. That is, under solvothermal conditions, the Schiff base reaction of polyamino monomers and polyaldehyde monomers takes advantage of the reversible cleavage-repair process of imine bonds to synthesize covalent organic frameworks with good crystallinity and ordered porous structures. Material.
[0040] The specific process is: add 10.5mg 5,5'-diamino-2,2'-bipyridine and 7.8mg 1,3,5-tris(4-aminophenyl)benzene into the reaction vessel, and add 1mL n-butyl Alcohol and dioxane mixed solvent (v / v=1:9) and 0.1mL acetic acid aqueous solution, cooled in...
Embodiment 2
[0069] Embodiment 2 relates to a preparation method of a cationic radical type covalent organic framework material, specifically as follows:
[0070] Step 1, preparation of covalent organic framework materials linked by imine bonds containing nitrogen heterocycles.
[0071] The specific process is: add 7.9mg 5,5'-diamino-2,3'-bipyridine and 12mg trihydroxy tritylaldehyde into the reaction vessel, and add 1.5mL n-butanol and o-dichlorobenzene mixed solvent (v / v=1:1) and 0.1mL acetic acid aqueous solution, cooled in 77K liquid nitrogen, freeze-thawed and degassed three times, and reacted in an oven at 100°C for 3 days after sealing the tube; Washing product I, which is a covalent organic framework material.
[0072] Step 2, preparation of ionized covalent organic framework material.
[0073] The specific process is: add 10 mg of product I and 10 mL of chloroform into the reaction vessel, stir at room temperature for 24 hours, after the reaction is completed, filter to obtain ...
Embodiment 3
[0077] Embodiment 3 relates to a preparation method of a cationic radical type covalent organic framework material, specifically as follows:
[0078] Step 1, preparation of covalent organic framework materials linked by imine bonds containing nitrogen heterocycles.
[0079] The specific process is: add 15mg of 5,5'-bis(4-aminophenyl)-2,2'-bipyridine and 12mg of 4,4'-biphenyldicarbaldehyde into the reaction vessel, and add 1mL of mesitylene and di Hexane mixed solvent (v / v=1:3) and 0.3mL pyrrolidine were cooled in 77K liquid nitrogen, freeze-thawed and degassed three times, and reacted in an oven at 110°C for 5 days after sealing the tube; after the reaction, The product I obtained by filtration and washed with acetone is a covalent organic framework material.
[0080] Step 2, preparation of ionized covalent organic framework material.
[0081] The specific process is: add 10 mg of product I and 10 mL of dibromomethane into the reaction vessel, stir at room temperature for 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com