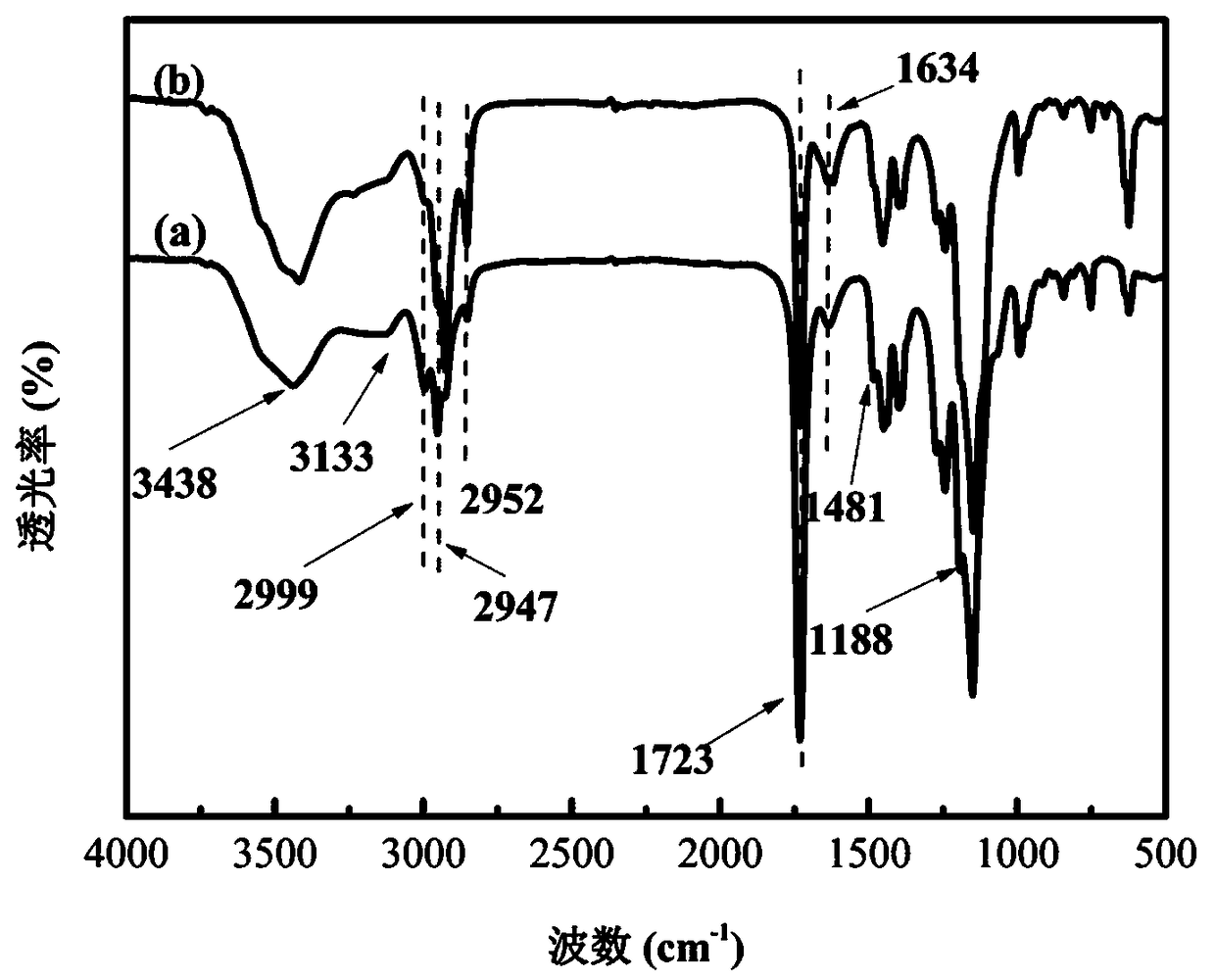
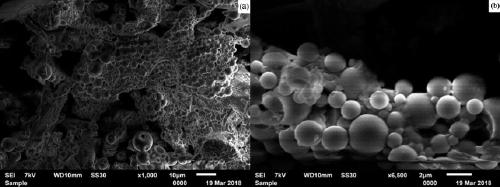
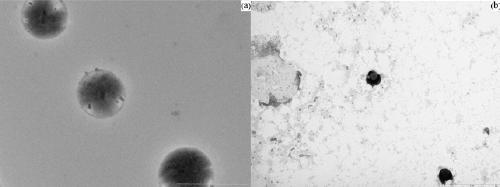
L-proline immobilized temperature-responsive core-shell microgel and preparation and application thereof
A temperature-responsive, core-shell microgel technology, which is applied in the preparation of organic compounds, catalytic reactions, chemical instruments and methods, etc., can solve the problem of reduced conversion rate, poor recycling performance of core-shell microgel catalysts, and difficult recovery. and other problems, to achieve high-efficiency recovery, improve recycling performance, and increase local concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] MMA and N-boc-L-ProlA were used as comonomers, AIBN was used as an initiator, and EGDMA was used as a crosslinking agent. Weigh 1.75g of MMA, 0.15g of N-boc-L-ProlA, 35.2mg of EGDMA, and 30mg of AIBN, disperse in 80mL solvent acetonitrile, transfer to a 500mL three-necked reaction flask, and stir until well mixed.
[0038] Install a fractionation column, a condenser tube and a receiving bottle, place the reaction bottle in an oil bath and heat slowly, make the reaction system boil at 90°C within 30 minutes, control the time to distill 40mL of acetonitrile within 1.5 hours, and end the reaction.
[0039] Put the reaction solution into a dialysis bag with a molecular weight cut-off of 3000, dialyze in deionized water for 72 hours, remove unreacted monomers, and freeze-dry to obtain the hydrophobic core microgel P(MMA-co-N-boc-L-ProlA) .
[0040] Take 1.0 g of the above-prepared microgel, dissolve it in 5 mL of dichloromethane, stir magnetically in an ice-water bath for...
Embodiment 2
[0055] Weigh 0.94g of MMA, 0.02g of N-boc-L-ProlA, 5.3mg of EGDMA, and 20mg of ABVN, disperse in 90mL solvent THF, transfer to a 250mL three-necked reaction flask, and stir until well mixed.
[0056] Install a distillation device, place the reaction bottle in an oil bath and heat slowly, so that the temperature of the reaction system is raised to 85°C within 40 minutes to boil, and the reaction is terminated after distilling 45mL of tetrahydrofuran within 2 hours under control.
[0057] Put the reaction solution into a dialysis bag with a molecular weight cut-off of 3000, dialyze in deionized water for 24 hours, remove unreacted monomers, and freeze-dry to obtain the hydrophobic core microgel P (MMA-co-N-boc-L-ProlA) .
[0058] Take 2.0 g of the above-prepared microgel, dissolve it in 10 mL of dichloromethane, stir magnetically in an ice-water bath for 20 min, slowly add 20 mL of a mixed solution of dichloromethane and trifluoroacetic acid with a volume ratio of 1:1, and react...
Embodiment 3
[0066] Weigh 6.0g of MMA, 0.1g of N-boc-L-ProlA, 256mg of EGDMA, and 100mg of AIBN, disperse them in 150mL of tetrahydrofuran as a solvent, transfer them into a 500mL three-necked reaction flask, and stir until they are evenly mixed.
[0067] Install a distillation device, place the reaction bottle in an oil bath and heat slowly, so that the temperature of the reaction system is raised to 85°C within 40 minutes to boil, and the reaction is terminated after controlling the time to distill 75mL of tetrahydrofuran within 2 hours.
[0068] Put the reaction solution into a dialysis bag with a molecular weight cut-off of 3000, dialyze in deionized water for 24 hours, remove unreacted monomers, and freeze-dry to obtain the hydrophobic core microgel P (MMA-co-N-boc-L-ProlA) .
[0069] Take 2.0 g of the above-prepared microgel, dissolve it in 10 mL of dichloromethane, stir magnetically in an ice-water bath for 20 min, slowly add 20 mL of a mixed solution of dichloromethane and trifluor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com