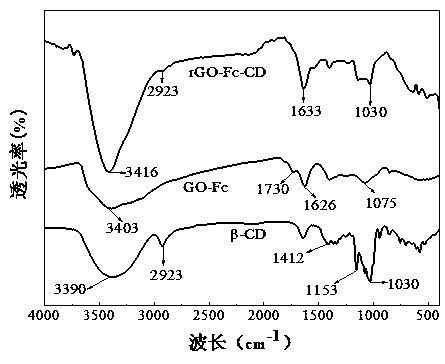
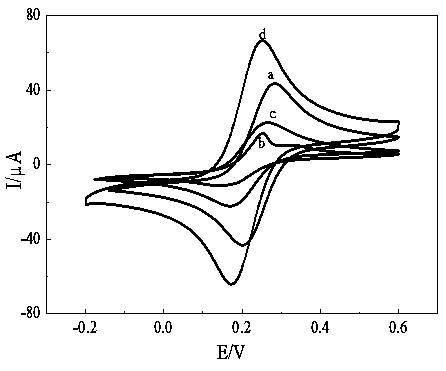
Preparation and application of graphene-ferrocene functionalized cyclodextrin chiral compound material
A composite material and graphene technology, which is applied in the preparation of β-cyclodextrin-based chiral composite materials and the preparation of cyclodextrin chiral composite materials, can solve problems such as low specific surface area and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation of chiral recognition material
[0034] (1) Preparation of graphene oxide (GO): Put expandable graphite into a crucible, place it in a muffle furnace, and heat it at 900-901°C for 30-50s to obtain expanded graphite. Put 1 g of expanded graphite into 500-501 mL of DMF, ultrasonicate for 24-25 h, wash and filter repeatedly with ethanol and water, and dry in vacuum at 60 °C to obtain exfoliated graphene. Put 1g of exfoliated graphite powder in a mixed solution of 90mL of concentrated sulfuric acid and 30mL of concentrated phosphoric acid, cool the temperature to about 0°C, then slowly add 10g of potassium permanganate, and control the reaction temperature below 5°C to prevent explosion. After the potassium permanganate was completely dissolved, the temperature was raised to 50°C and kept stirring at this temperature for 12h. After the reaction, the system was cooled to room temperature, and 200mL of ice water and 5mL of 30% hydrogen peroxide ...
Embodiment 2
[0037] Example 2. Construction of rGO-Fc-CD / GCE and chiral recognition of tryptophan isomers
[0038] Construction of chiral electrochemical sensor rGO-Fc-CD / GCE: uniformly disperse rGO-Fc-CD in water to form a new 1mg / mL dispersion, then take 8~9μL of 1mg / mL rGO-Fc-CD to disperse The droplets were coated on the surface of glassy carbon electrode (GCE) and dried to construct rGO-Fc-CD / GCE.
[0039] Chiral recognition of phenylalanine isomers: Immerse rGO-Fc-CD / GCE in 20mL 5mM / L of L-phenylalanine (L-Phe) and D-phenylalanine (D-Phe) isomers In vivo, differential pulse voltammetry was used for identification: the scanning potential was -0.2~0.6V, the pulse amplitude was 50mV, and the pulse width was 0.1s. Due to the interaction of L-phenylalanine and D-phenylalanine with rGO-Fc-CD / GCE, the peak current value of D-phenylalanine is larger, and the peak current value of L-phenylalanine is smaller, so that it can Recognition of phenylalanine isomers, recognition efficiency: I D ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com