Synthesis method and application of compound
A synthesis method and compound technology are used in the synthesis of methyl 2-benzene-1,3,5-tricarboxylate and the synthesis of pesticide intermediate impurities, which can solve the problems of low reaction yield and the like, and achieve a simple operation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Synthetic method of 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylic acid methyl ester
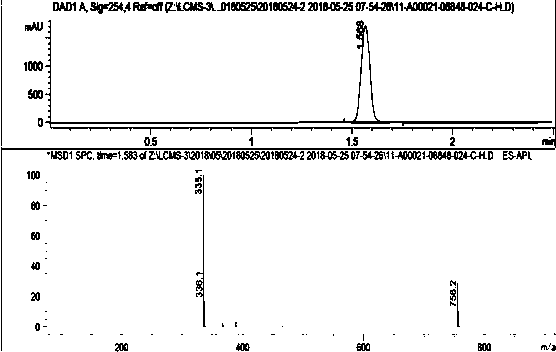
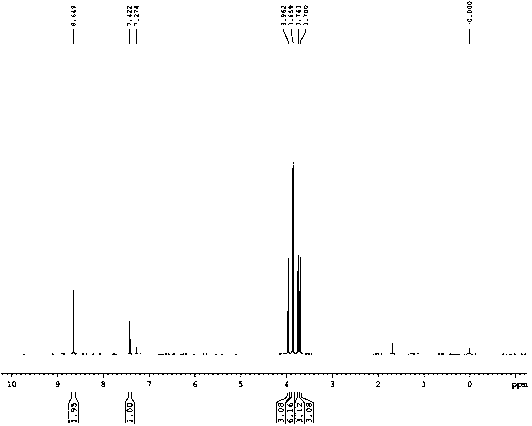
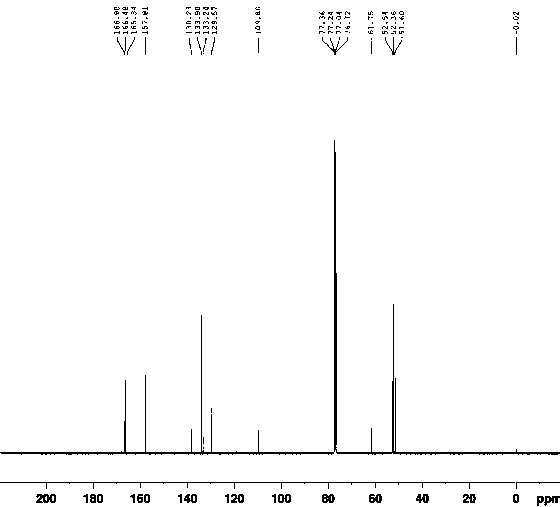
[0049]Add 540g of sodium methoxide into the reaction vessel at room temperature, add 1160g of methyl 3-methoxyacrylate into the reaction vessel, start stirring for about 10 minutes, add 8L of solvent DMAC, heat to the reaction temperature of 35°C, and react for 2.5 hours. After the reaction was completed, the reaction solution was added into ice water with 4 times the amount of solvent (by volume), stirred for 20 min, and solids were precipitated and filtered. The filtrate was extracted with ethyl acetate, and the organic phase was rotary evaporated. After cooling at low temperature, a white solid precipitated, and filtered to obtain methyl 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylate. For its related detection spectrum, see figure 1 - Figure 10 .
Embodiment 2-9
[0050] Example 2-9 Synthetic method of 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylic acid methyl ester
[0051] Examples 2 to 9 are respectively a synthesis method of 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylic acid methyl ester , their operating steps are all similar to those in Example 1, only the type and amount of the reactant, and the control parameters in the process, the yield of the product, etc. are different, specifically see the following table:
[0052]
Embodiment 10
[0053] Example 10 Application of methyl 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylate
[0054]
[0055] In the process of synthesizing the intermediate of flonicamid by the method of the above formula, the impurity 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3 , 5-methyl tricarboxylate, and the presence of impurities will not only affect the quality of intermediates and final products, but also greatly reduce the yield of the reaction.
[0056] In this example, 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylic acid methyl ester prepared in Example 1-9 Provides an application that can be used as a standard reference substance to detect flonicamid and its intermediate N-(2-methoxycarbonylvinyl)-4,4,4-trifluoro-3-one-1-butan Whether and how much methyl 2-(1,3-dimethoxy-3-oxoprop-1-en-2-yl)benzene-1,3,5-tricarboxylate exists in enamine. In addition, in the process of synthesizing the intermediate of flonicamid with the above method, it ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com