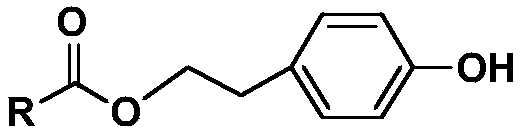
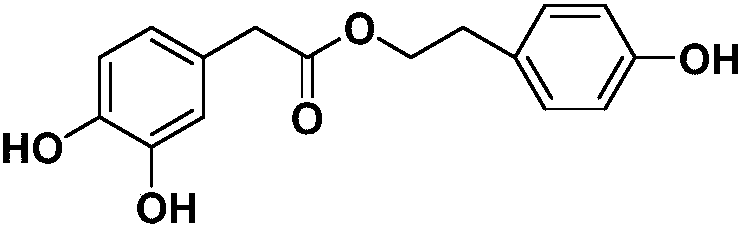
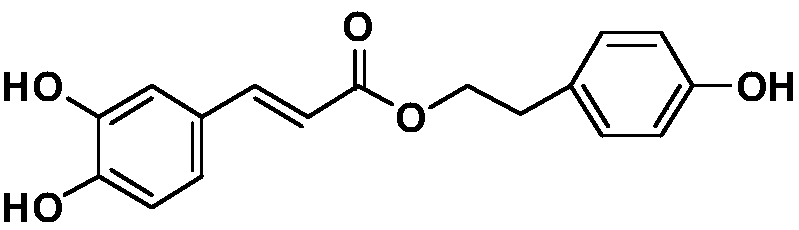
Preparation method and application of tyrosol phenolate compound
A technology of tyrosol phenolate and tyrosol phenate, which is applied in the field of preparation of tyrosol phenate compounds, can solve the problems of high reactivity of phenolic acid chloride, low yield of target substance, instability and easy decomposition, etc. Achieve the effects of improving pharmacokinetics, high yield, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of 3,4-dihydroxybenzoic acid-4-hydroxyphenethyl ester is:
[0025] Continuously feed nitrogen into the reactor to protect the reaction, sequentially add 4mmol tyrosol, 4mmol triphenylphosphine and 4mmol 3,4-dihydroxybenzoic acid, then add 10mL tetrahydrofuran, and add 6mmol dihydrofuran dropwise at 0°C. Diisopropyl nitrogen dicarboxylate, remove the ice bath after the dropwise addition, heat up to room temperature and stir for 20 hours to react. After the reaction is completed, seal and evaporate the reaction solution to dryness, dissolve it in 140mL ethyl acetate and wash it with 150mL saturated aqueous sodium bicarbonate solution for 3 times, and then washed 3 times with 150mL saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, desalted by suction filtration, and concentrated to obtain a concentrate; the concentrate was subjected to column chromatography with dichloromethane as a solvent, and 3,4- Dihydroxybenzoic ac...
Embodiment 2
[0027] The preparation method of 3,5-dihydroxybenzoic acid-4-hydroxyphenethyl ester is:
[0028] at N 2 Weigh 4 mmol of tyrosol under protection and put it in a reaction flask, then add 4 mmol of triphenylphosphine, then add 5 mmol of 3,5-dihydroxybenzoic acid, add 10 mL of tetrahydrofuran, add 5 mmol of azodicarboxylate dropwise under stirring at 0°C Isopropyl ester, react at room temperature for 40 hours after the drop, evaporate the reaction solution to dryness after the reaction, add 150mL ethyl acetate to dissolve, wash 3 times with 140mL saturated aqueous sodium bicarbonate solution, wash 3 times with 140mL saturated aqueous sodium chloride solution, anhydrous Dry over sodium sulfate, remove salt by suction filtration, evaporate the solvent under reduced pressure and purify by gel column chromatography to obtain a white solid which is the target compound with a yield of 68%.
Embodiment 3
[0030] The preparation method of 2,3-dihydroxybenzoic acid-4-hydroxyphenethyl ester is:
[0031] Under the protection of nitrogen, add 4mmol tyrosol to the reactor, then add 6mmol triphenylphosphine, 4mmol 2,3-dihydroxybenzoic acid and 12mL tetrahydrofuran in sequence, and add 6mmol diisoazodicarboxylate dropwise at 0°C. Propyl ester, after the dropwise addition, warm up to room temperature, stir and react for 24 hours, evaporate the reaction solution to dryness after the reaction, dissolve the residue with 140mL ethyl acetate, and then use 144mL saturated aqueous sodium bicarbonate solution, 144mL saturated sodium chloride The aqueous solution was washed 4 times respectively, dried with anhydrous sodium sulfate, filtered to remove salt, and concentrated to obtain a concentrate; a mixture prepared with dichloromethane and methanol at a volume ratio of 1:1 was used as a solvent, and the concentrate was subjected to column chromatography , and isolated 2,3-dihydroxybenzoic acid-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com