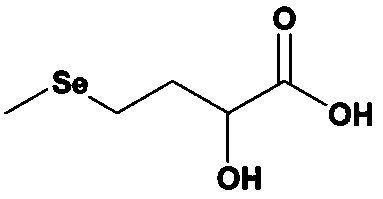
Preparation method of selenomethionine hydroxy analogue
A technology of selenomethionine hydroxyl and analogs, which is applied in the fields of amino acid feed, food and medicine, and can solve the problems of small income, unreported preparation method, difficult management and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Preparation of hydrogen selenide: In the hydrogen selenide manufacturing device, 5kg of metal selenium is put into the reaction furnace, and the reaction furnace is heated to 500 ° C. At the same time, the circulating hydrogen is combined with the hydrogen from the hydrogen source, thereby The flow is put into the reactor. The temperature of the heater / cooler on the hydrogen injection side for heating operation was set to 300°C, and the temperature of the heater / cooler on the reaction gas side for cooling operation was set to 100°C. In addition, the temperature of the hydrogen selenide trap was set at -196°C. When the capture amount of hydrogen selenide in the hydrogen selenide catcher reaches 1kg, switch the heating operation and cooling operation of the heating / cooler, and the switching of this heating and cooling operation will be performed according to the hydrogen selenide capture amount every time it reaches 1kg. The mode of switching is repeated, and the hydroge...
Embodiment 1
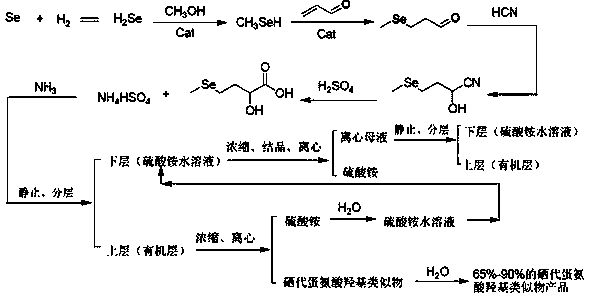
[0056] A preparation method of selenomethionine hydroxyl analogs, comprising the following steps:
[0057] Step (1): Selenium powder is reacted with hydrogen to obtain hydrogen selenide, and hydrogen selenide is reacted with methanol under the action of a catalyst to obtain methylselenol;
[0058] Step (2): adding methylselenol obtained in step (1) to acrolein to obtain methylselenopropionaldehyde;
[0059] Step (3): The methylselenopropionaldehyde obtained in step (2), water, and deaminated hydrocyanic acid gas mixture are subjected to a cyanidation addition reaction in the presence of a catalyst to obtain DL-2-hydroxyl -4-Methylselenobutyronitrile;
[0060] Step (4): Mix the DL-2-hydroxy-4-methylselenobutyronitrile obtained in step (3) with sulfuric acid for hydrolysis. After the hydrolysis is complete, add ammonia to neutralize ammonium bisulfate, stand still, separate Obtained: the upper layer contains an organic layer of selenomethionine hydroxyl analogue, and the lower...
Embodiment 2
[0077] A preparation method of selenomethionine hydroxyl analogs, comprising the following steps:
[0078] Step (1): Selenium powder is reacted with hydrogen to obtain hydrogen selenide, and hydrogen selenide is reacted with methanol under the action of a catalyst to obtain methylselenol;
[0079] Step (2): adding methylselenol obtained in step (1) to acrolein to obtain methylselenopropionaldehyde;
[0080] Step (3): The methylselenopropionaldehyde obtained in step (2), water, and deaminated hydrocyanic acid gas mixture are subjected to a cyanidation addition reaction in the presence of a catalyst to obtain DL-2-hydroxyl -4-Methylselenobutyronitrile;
[0081] Step (4): Mix the DL-2-hydroxy-4-methylselenobutyronitrile obtained in step (3) with sulfuric acid for hydrolysis. After the hydrolysis is complete, add ammonia to neutralize ammonium bisulfate, stand still, separate Obtained: the upper layer contains an organic layer of selenomethionine hydroxyl analogue, and the lower...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com