Thiazolyl aminobenzoic acid derivative and application thereof
An alkyl and methyl technology, applied in the field of preparation of thiazole aminobenzoic acid derivatives, can solve problems such as inability to effectively solve drug resistance, and achieve the effects of solving drug resistance problems, inhibiting cancer cells, and improving the strength of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
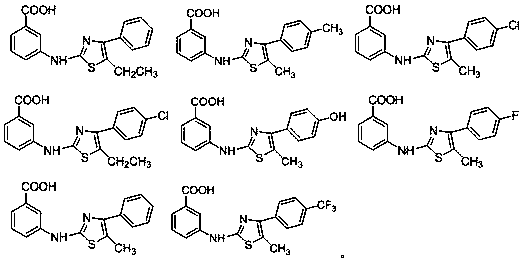
[0045] Example 1 3-[(5-Ethyl-4-phenylthiazol-2-yl)amino]benzoic acid
[0046] Step a:
[0047]
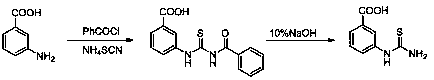
[0048] Add 11.4341 g (0.12 mol) of ammonium thiocyanate and 20 mL of acetone into a 100 mL oblique-necked reaction flask equipped with mechanical stirring and a condenser, and stir evenly through mechanical stirring. 16.8034 g (0.13 mol) of benzoyl chloride was added dropwise (dropped in 10 min), and the solution changed from clear to white turbid. Heat to reflux, add 14.1147 g (0.10 mol) m-aminobenzoic acid in 4 batches, monitor the reaction process by TLC (ethyl acetate:petroleum ether = 4:1), and complete the reaction in 8 h. Cool, filter, and dry the obtained solid to obtain 28.0041 g of light yellow powder 3-(3-benzoylthioureido)benzoic acid, m.p. 184 ~ 186 ℃.
[0049] Add 0.9913 g (0.12 mol) of 3-(3-benzoylthioureido)benzoic acid and 33 mL of 10% NaOH into a 100 mL oblique-necked reaction flask with a condenser, the measured pH=13, magnetic stirring, and heating Reflux,...
Embodiment 2
[0053] Example 2 3-[(5-methyl-4-(4-methylaryl)thiazol-2-yl)amino]benzoic acid
[0054]
[0055] The operation was the same as in Example 1, and 2.4358 g of dark green crystals were weighed, with a yield of 57.72%, m.p. 257-258 °C. 1 H NMR (DMSO-D 6 , 400 MHz), δ : 2.35 (s, 3H, CH 3 ), 2.42 (s, 3H, CH 3 ), 7.27-8.27 (m, 8H, 2×C 6 h 4 ), 10.42 (s, 1H, COOH).
Embodiment 3
[0056] Example 3 3-[(4-(4-chlorophenyl)-5-methylthiazol-2-yl)amino]benzoic acid
[0057]
[0058] The operation was the same as that in Example 1 to obtain 4.5789 g of brown powder with a yield of 54.19%, m.p. 257-258°C. 1 HNMR (DMSO-D 6 , 400 MHz), δ : 2.44 (s, 3H, CH 3 ), 7.44-8.27 (m, 8H, 2×C 6 h 4 ), 10.40 (s, 1H, COOH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com