Sesquiterpene compound and preparation method and application thereof
A compound, sesquiterpene technology, applied in the field of pinane-type sesquiterpenoids and their separation and purification, can solve the problems of no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
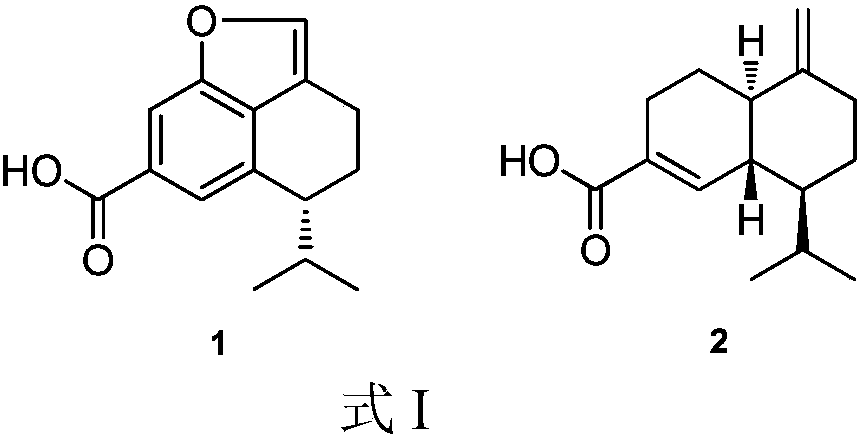
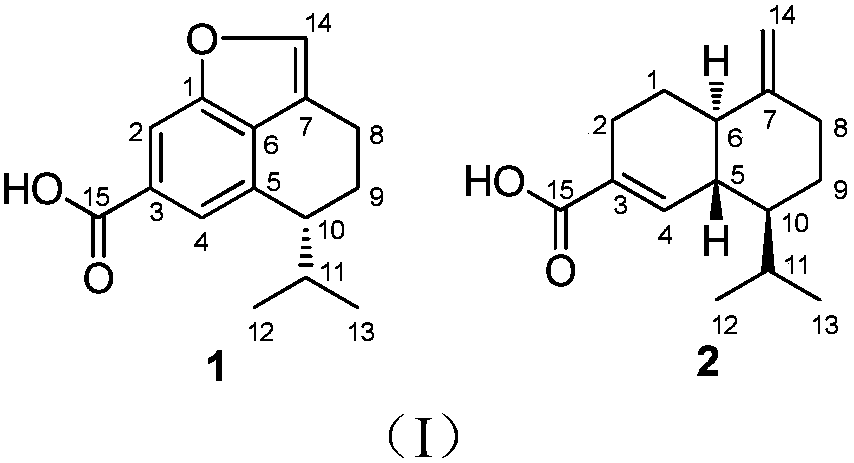
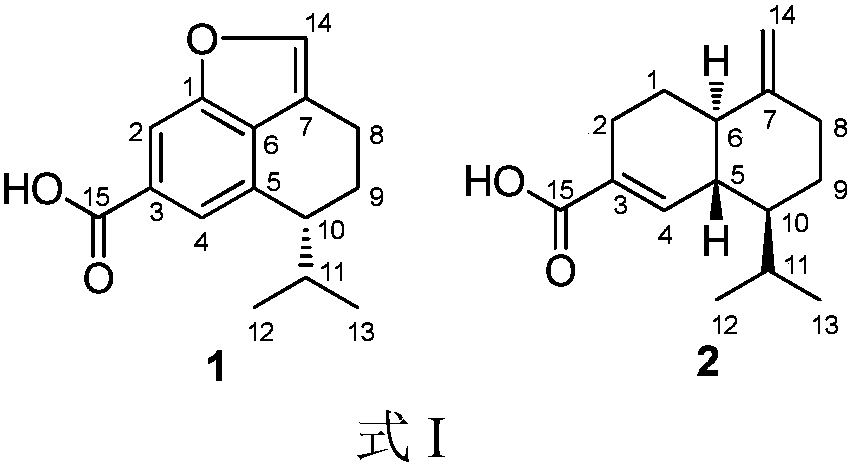
[0023] The preparation method of compound 1 and 2 shown in embodiment 1. formula I
[0024] With Trichoderma virens gained according to above-mentioned literature or public circulation channel, get the Trichoderma virens (size 2.0 centimeters * 2.0 centimeters) that grows in the PDA plate culture medium and inoculate in the rice solid culture medium that has sterilized, Standing culture at room temperature for 30 days, the fermentation product was soaked and extracted 3 times with ethyl acetate, and the combined extracts were concentrated to obtain a fermented crude extract;
[0025] The formula of the rice solid medium is: every 100 milliliters of distilled water contains 70 grams of rice, 0.2 grams of corn steep liquor, and 0.3 grams of peptone;
[0026] The crude extract was subjected to vacuum silica gel column chromatography, and the petroleum ether-ethyl acetate with a gradient of 20:1 to 1:1 (v / v, the same below) and the gradient of 20:1 to 1:1 dichloromethane- Methano...
Embodiment 2
[0037] Example 2. Bacteriostatic activity
[0038] The antibacterial activity of compounds 1 and 2 shown in formula I was detected by the minimum inhibitory concentration method. The following bacterial strains were selected for antibacterial activity test: human pathogenic bacteria (1 strain): Escherichia coli (Escherichia coli); aquatic pathogenic bacteria (10 strains): Aeromonas hydrophilia (Aeromonas hydrophilia), catfish Edwardsiella (Edward megi), Edwardsiella tarda, Micrococcus luteus, Pseudomonasaeruginosa, Vibrio alginolyticus, V. anguillarum, Harvestella Vibrio harveyi, V.parahemolyticus, V.vulnificus; agricultural disease fungi (15 strains): tomato early blight (Alternaria solani), wheat root rot (Bipolarissorokiniana ), Ceratobasidium cornigerum, Coniothyrium diplodiella, Colletottichum gloeosporioides, Colletottichum gloeosporioides Penz, Fusarium graminearum, Pepper root rot (Fusarium solani), Fusarium oxysporum, Fusarium oxysporum. sp. cucumebrium Owen, Fusari...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com