A kind of ferrocene derivative and composition thereof that can be used as photoredox catalyst in photopolymerization
A technology of ferrocene derivatives and compositions, applied in the field of organometallic photofunctional molecule synthesis and visible light photopolymerization, which can solve the problems of irreplaceability and achieve good redox ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
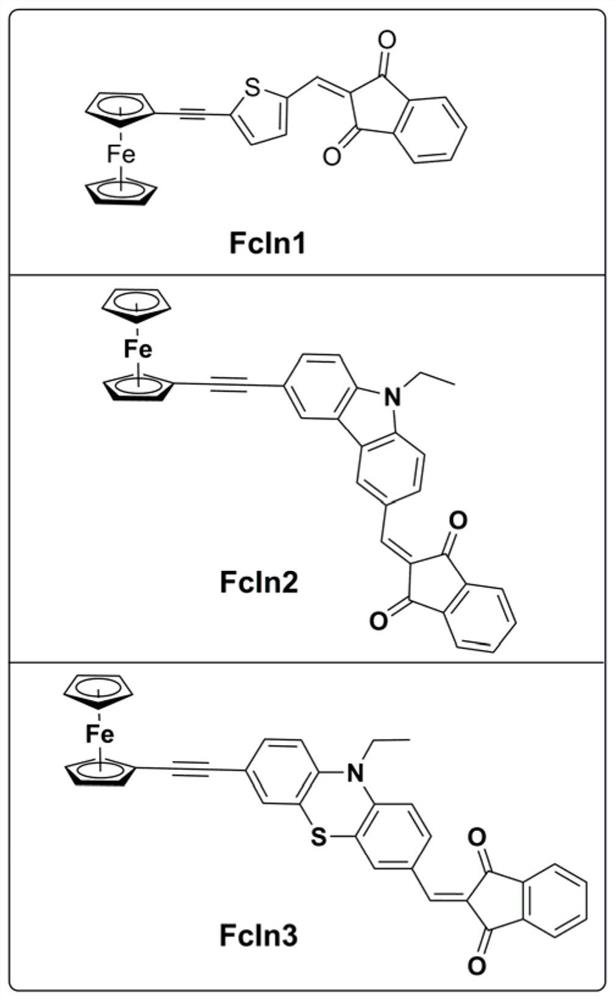
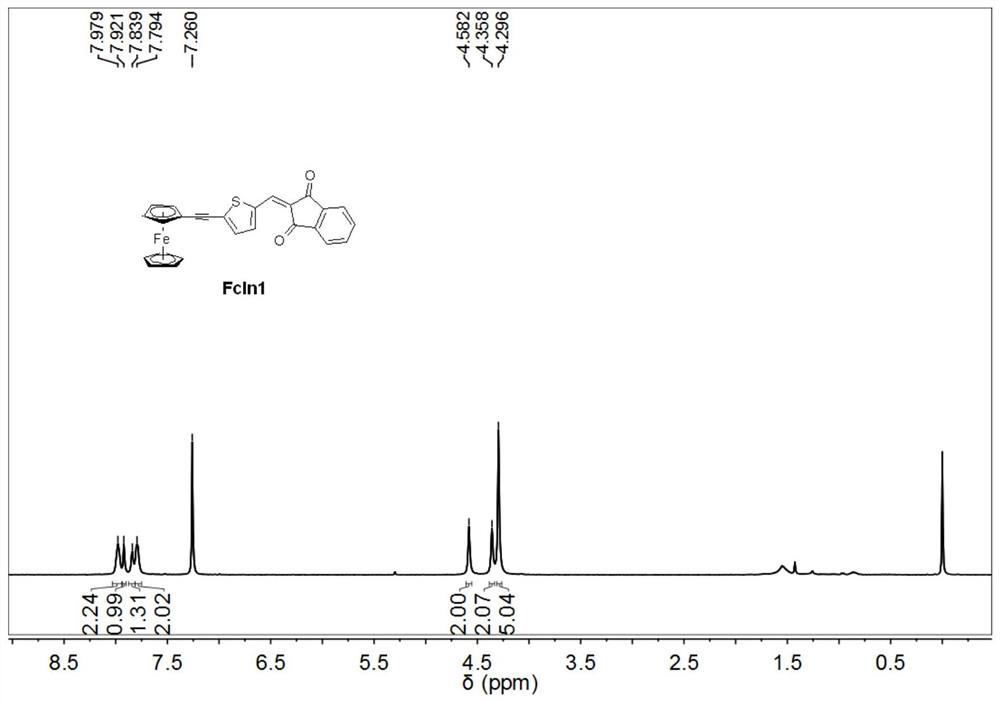
[0035] Synthesis of ferrocene derivative FcIn1:
[0036]
[0037] In 10 mL of DMF, add 0.19 g (1 mmol) 5-bromo-2-thiophene aldehyde, 0.32 g (1.5 mmol) ethynyl ferrocene, 21 mg (0.03 mmol) Pd(PPh 3 ) 2 Cl 2 , 10 mg (0.05 mmol) of cuprous iodide, 13.12 mg (0.05 mmol) of triphenylphosphine, and 1 ml of triethylamine were magnetically stirred in an oil bath at 90° C. for 9 hours under nitrogen protection. After the completion of the reaction was monitored by thin-layer chromatography, the reactant was added to water to dilute to precipitate a solid, and the stirring was continued for 1 hour. The solid was collected and purified by column chromatography to obtain a red solid. 0.31 g of the obtained red solid and 0.22 g of 1,3-indanedione were dissolved in 6 mL of a solvent with a volume ratio of toluene / methanol of 1:1, and then 0.1 mL of piperidine was added. The resulting reaction was magnetically stirred in an 80°C oil bath for 4 hours. After the completion of the reacti...
Embodiment 2
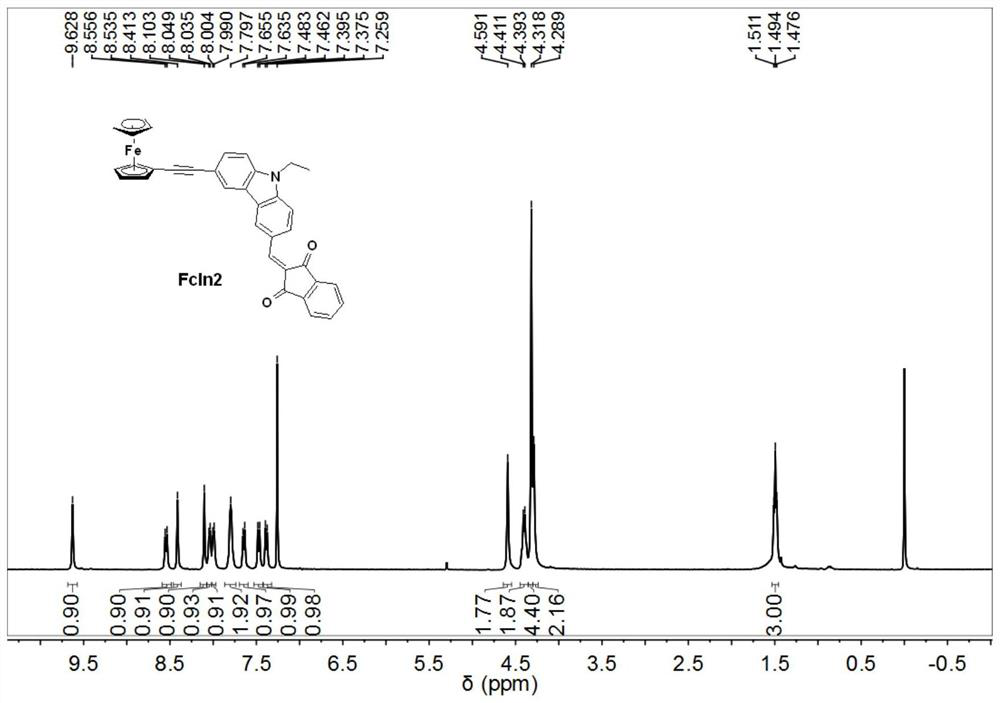
[0039] Synthesis of ferrocene derivative FcIn2:
[0040]
[0041] In 10 mL of DMF, add 0.3 g (1 mmol) 9-ethyl-6-bromo-carbazole aldehyde, 0.32 g (1.5 mmol) ethynylferrocene, 21 mg (0.03 mmol) Pd(PPh 3 ) 2 Cl 2 , 10 mg (0.05 mmol) of cuprous iodide, 13.12 mg (0.05 mmol) of triphenylphosphine, and 1 ml of triethylamine were magnetically stirred in an oil bath at 90° C. for 9 hours under nitrogen protection. After the completion of the reaction was monitored by thin-layer chromatography, the reactant was added to water to dilute to precipitate a solid, and the stirring was continued for 1 hour. The solid was collected and purified by column chromatography to obtain a red solid. 0.31 g of the obtained red solid and 0.22 g of 1,3-indanedione were dissolved in 6 mL of a solvent with a volume ratio of toluene / methanol of 1:1, and then 0.1 mL of piperidine was added. The resulting reaction was magnetically stirred in an 80°C oil bath for 4 hours. After the completion of the reac...
Embodiment 3
[0043] Synthesis of ferrocene derivative FcIn3:
[0044]
[0045] Add 0.33g (1mmol) 10-ethyl-7-bromo-3-phenothiazinaldehyde, 0.32g (1.5mmol) ethynylferrocene, 21mg (0.03mmol) Pd(PPh) to 10mL of DMF 3 ) 2 Cl 2 , 10 mg (0.05 mmol) of cuprous iodide, 13.12 mg (0.05 mmol) of triphenylphosphine, and 1 ml of triethylamine were magnetically stirred in an oil bath at 90° C. for 9 hours under nitrogen protection. After the completion of the reaction was monitored by thin-layer chromatography, the reactant was added to water to dilute to precipitate a solid, and the stirring was continued for 1 hour. The solid was collected and purified by column chromatography to obtain a red solid. 0.31 g of the obtained red solid and 0.22 g of 1,3-indanedione were dissolved in 6 mL of a solvent with a volume ratio of toluene / methanol of 1:1, and then 0.1 mL of piperidine was added. The resulting reaction was magnetically stirred in an 80°C oil bath for 4 hours. After the completion of the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com