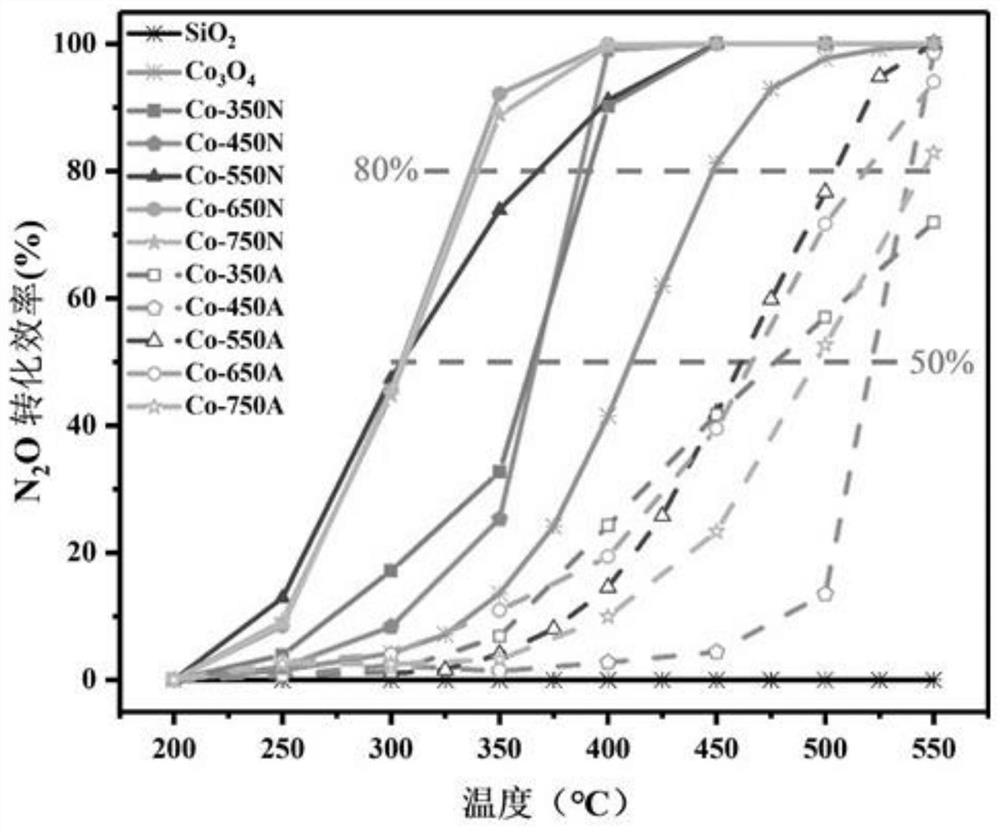
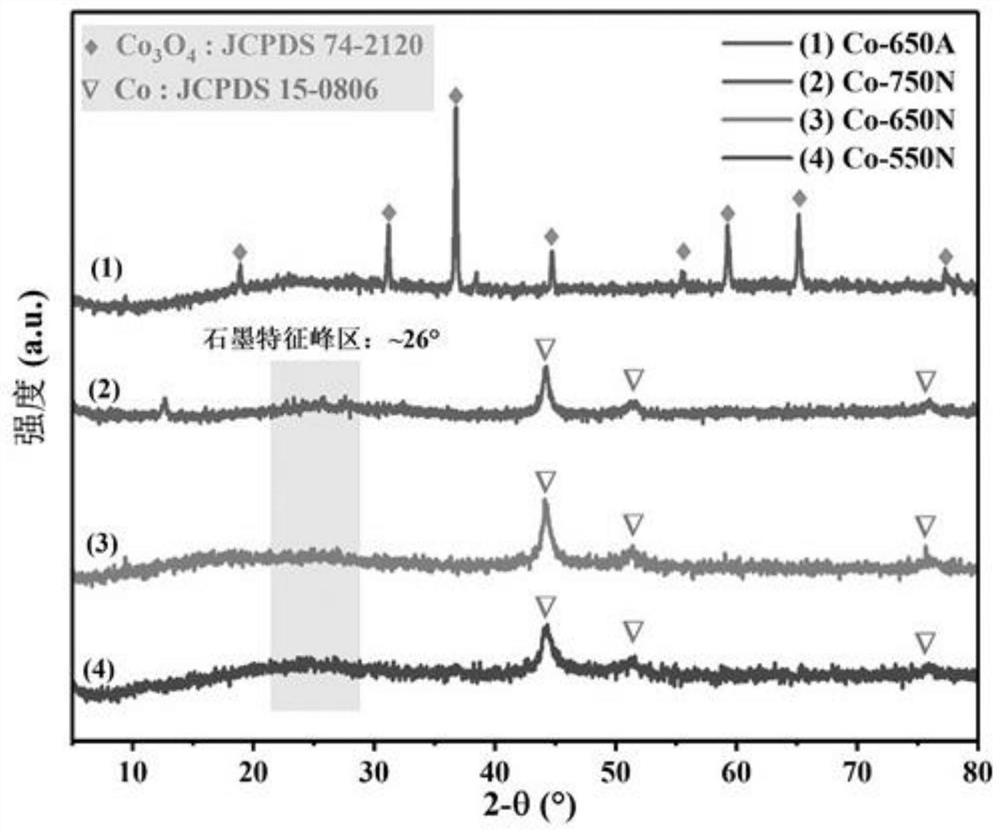
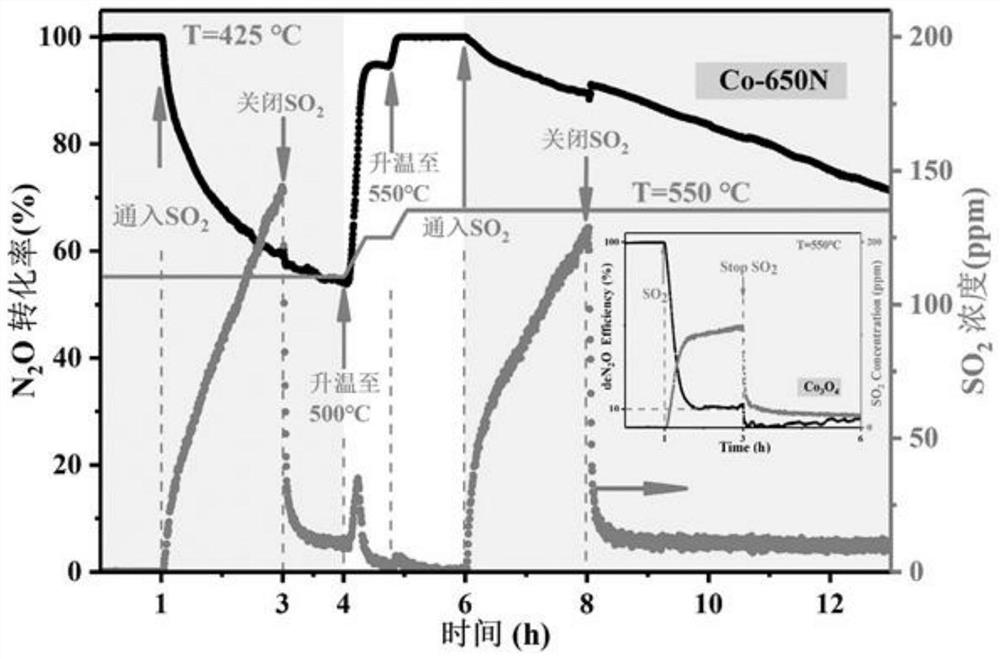
Preparation method of cobalt-based metal organic framework derivative catalyst for catalytic decomposition of N2O
An organic framework, catalytic decomposition technology, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, separation methods, etc., to achieve the effects of excellent activity, high preparation cost, and excellent redox performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Weigh 0.025mol of 2-methylimidazole and 0.025mol of triethylamine and dissolve them in 250ml of deionized water. Stir magnetically and sonicate for 20 minutes until the drug is completely dissolved. Slowly introduce 250mL dissolved with 0.01mol Co(NO 3 ) 2 ·6H 2 O solution, after standing at room temperature for 10 minutes, the solution was centrifuged at a high speed (5000r / min) and washed. The collected solid precipitate was dried in an oven at 110 °C for 6 h to obtain the precursor ZIF-67. The precursor was further heated to 650°C at a heating rate of 2°C / min in nitrogen, and then annealed after pyrolysis at 650°C for 3 hours to obtain the cobalt nanocomposite catalyst Co-650N supported on the porous carbon material.
Embodiment 2
[0034] The precursor ZIF-67 in Example 1 was heated to 550°C at a heating rate of 2°C / min in nitrogen gas, and then pyrolyzed for 3 hours. After annealing, the cobalt nanocomposite catalyst Co-550N supported on the porous carbon material was obtained. .
Embodiment 3
[0036] The precursor ZIF-67 in Example 1 was heated to 750°C at a heating rate of 2°C / min in nitrogen, and then pyrolyzed for 3 hours. After annealing, the cobalt nanocomposite catalyst Co-750N supported on the porous carbon material was obtained. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com