Preparation method of lithium zirconate material capable of absorbing high-temperature carbon dioxide
A technology of carbon dioxide and lithium zirconate, applied in chemical instruments and methods, separation methods, inorganic chemistry, etc., can solve the problems of high synthesis cost and poor product performance, and achieve the effects of excellent absorption performance, low cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The reaction raw materials are zirconium oxynitrate, lithium hydroxide and ammonium bicarbonate, and the lithium zirconate high-temperature adsorbent is prepared by a precipitation method in an ammonia solution. ZrO(NO 3 ) 2 Dissolve in deionized water, add ammonia water according to the ammonia concentration in the reaction system of 0.8mol / L, weigh a certain amount of LiOH·H 2 O, drop LiOH and ammonium bicarbonate solution into ZrO(NO 3 ) 2 solution, stirred for 1 h after titration, then evaporated to dryness, placed the obtained material in an oven, and dried at a constant temperature at 100°C for 12 h in an air atmosphere to obtain a lithium zirconate precursor. After grinding the obtained precursor powder evenly, put In a tube furnace, raise the temperature to 550°C at a rate of 5°C / min and hold for 6 hours to obtain the lithium zirconate material.
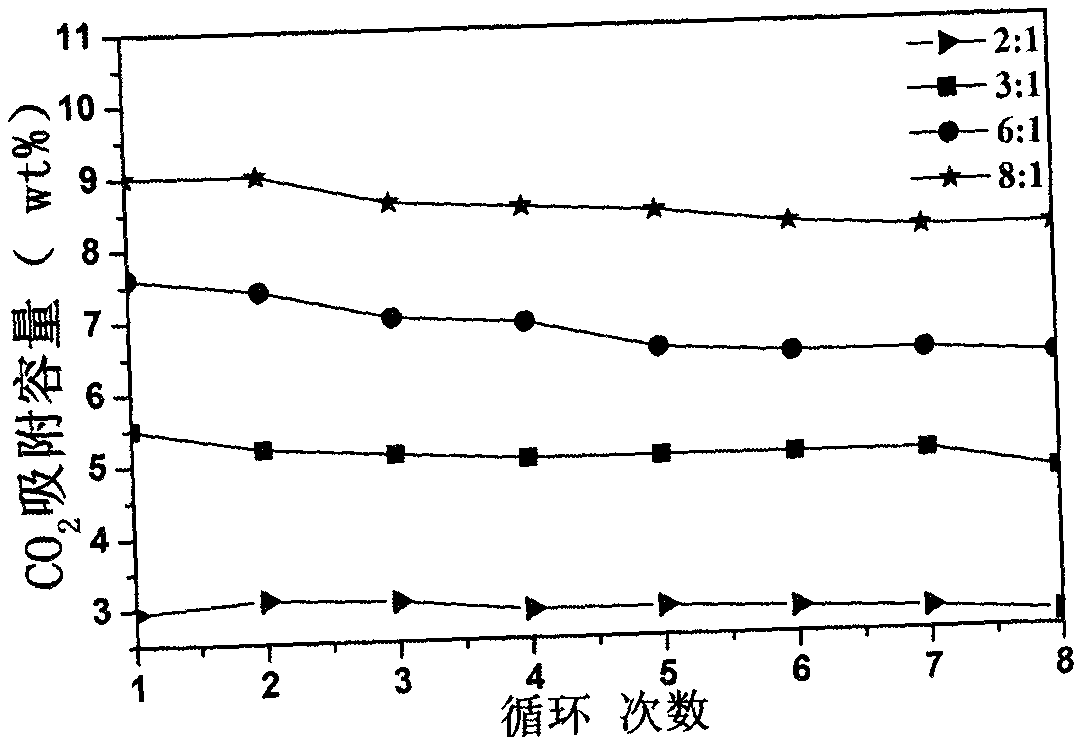
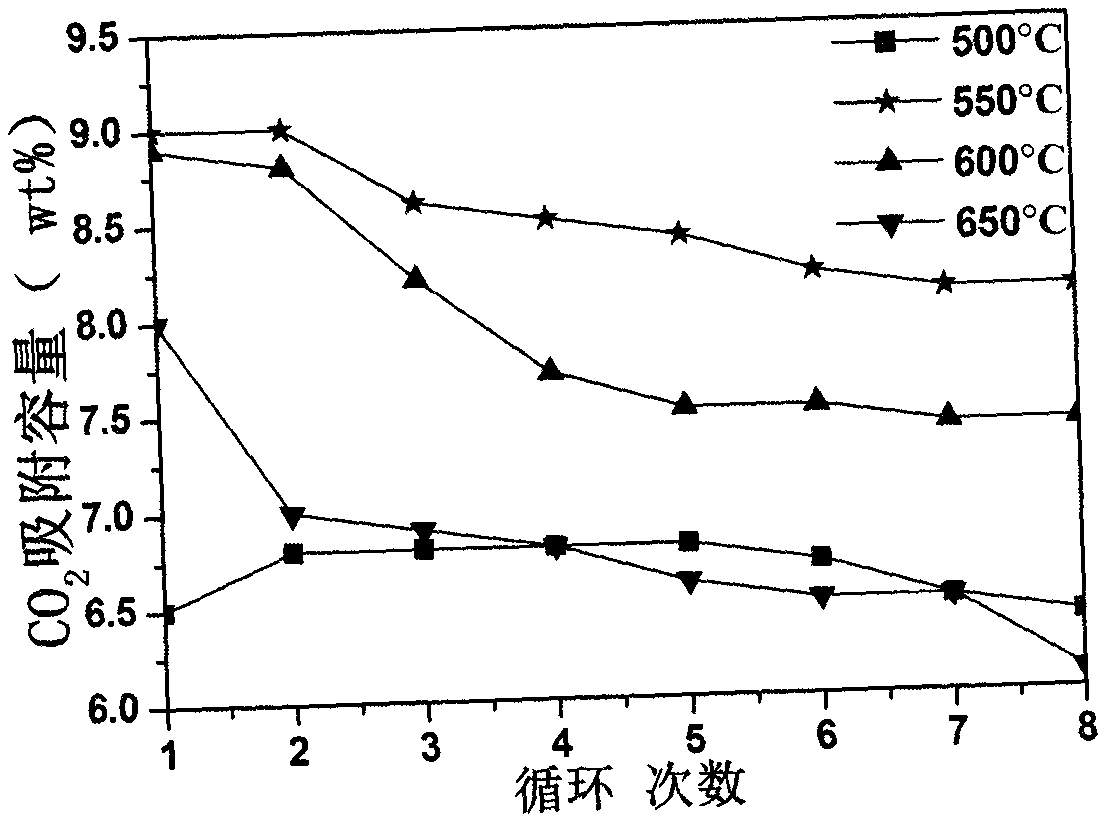
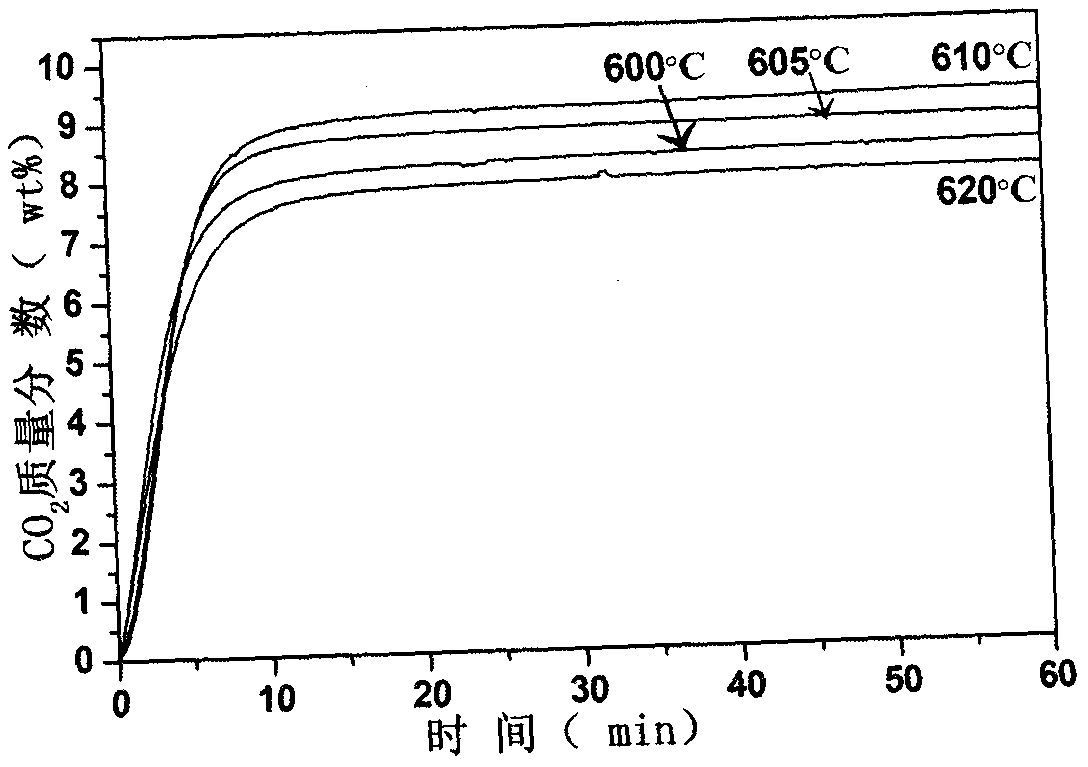
[0020] The synthesized lithium zirconate material was placed in a thermogravimetric analyzer under CO 2 :N 2 =...
Embodiment 2
[0022] The reaction raw materials are zirconium oxynitrate, lithium hydroxide and sodium bicarbonate, and the lithium zirconate high-temperature adsorbent is prepared by a precipitation method in an ammonia solution. ZrO(NO 3 ) 2 Dissolve in deionized water, add ammonia water according to the ammonia concentration in the reaction system is 0.8mol / L, weigh a certain amount of LiOH·H 2 O, drop LiOH and ammonium bicarbonate solution into ZrO(NO 3 ) 2 solution, stirred for 1 h after titration, then evaporated to dryness, placed the obtained material in an oven, and dried at a constant temperature at 100°C for 12 h in an air atmosphere to obtain a lithium zirconate precursor. After grinding the obtained precursor powder evenly, put In a tube furnace, raise the temperature to 550°C at a rate of 5°C / min and hold for 6 hours to obtain the lithium zirconate material.
[0023]The synthesized lithium zirconate material was placed in a thermogravimetric analyzer under CO 2 :N 2 =1:7...
Embodiment 3
[0025] The reaction raw materials are zirconium oxynitrate, lithium hydroxide and ammonium bicarbonate, and the lithium zirconate high-temperature adsorbent is prepared by a precipitation method in an ammonia solution. ZrO(NO 3 ) 2 Dissolve in deionized water, add ammonia water according to the ammonia concentration in the reaction system of 0.8mol / L, weigh a certain amount of LiOH·H 2 O, drop LiOH and ammonium bicarbonate solution into ZrO(NO 3 ) 2 solution, stirred for 1 h after titration, then evaporated to dryness, placed the obtained material in an oven, and dried at a constant temperature at 100°C for 12 h in an air atmosphere to obtain a lithium zirconate precursor. After grinding the obtained precursor powder evenly, put In a tube furnace, raise the temperature to 550°C at a rate of 5°C / min and hold for 6 hours to obtain the lithium zirconate material.
[0026] The synthesized lithium zirconate material was placed in a thermogravimetric analyzer under CO 2 :N 2 =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com