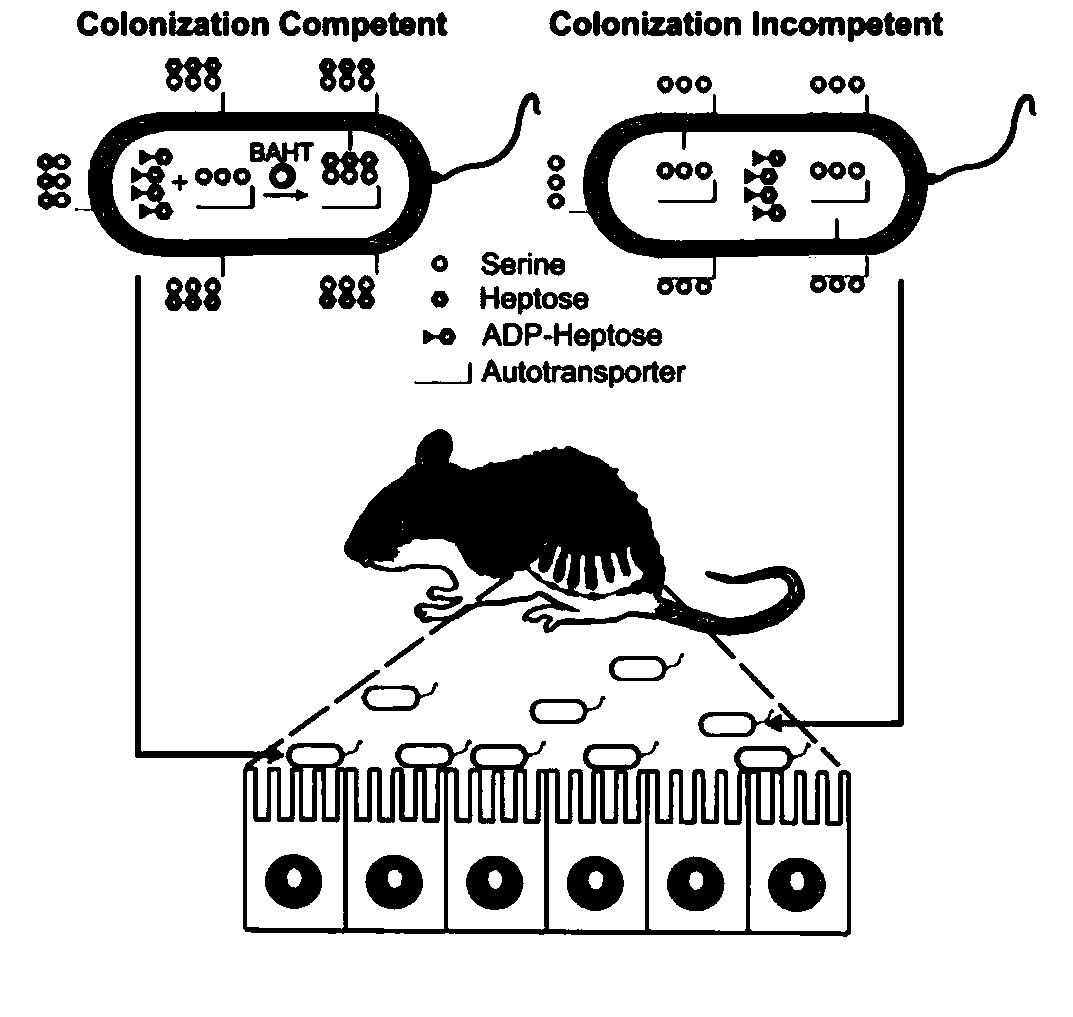
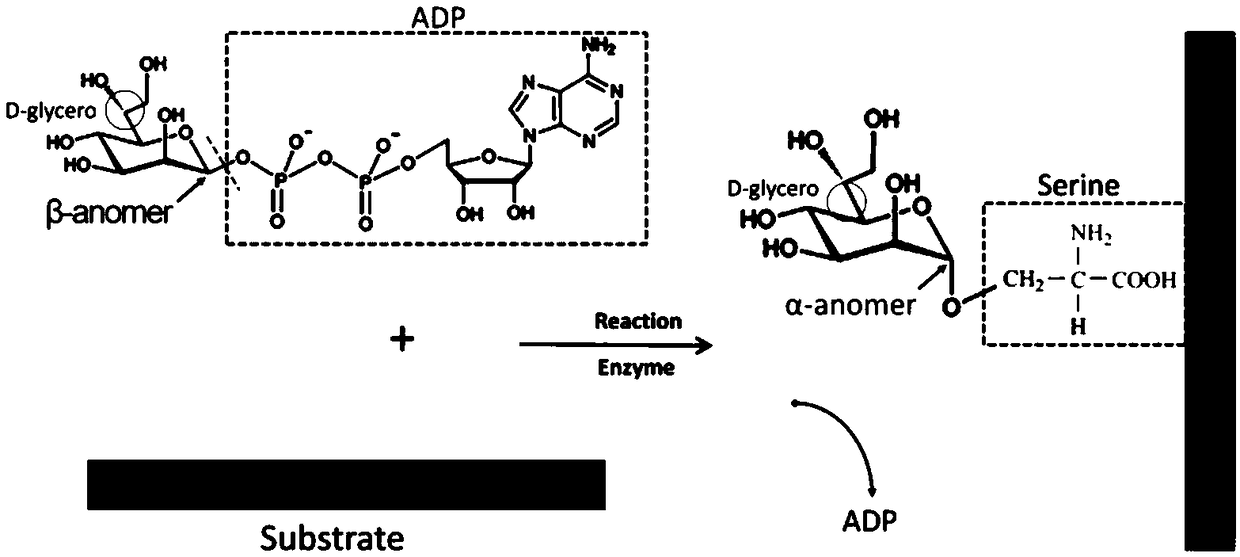
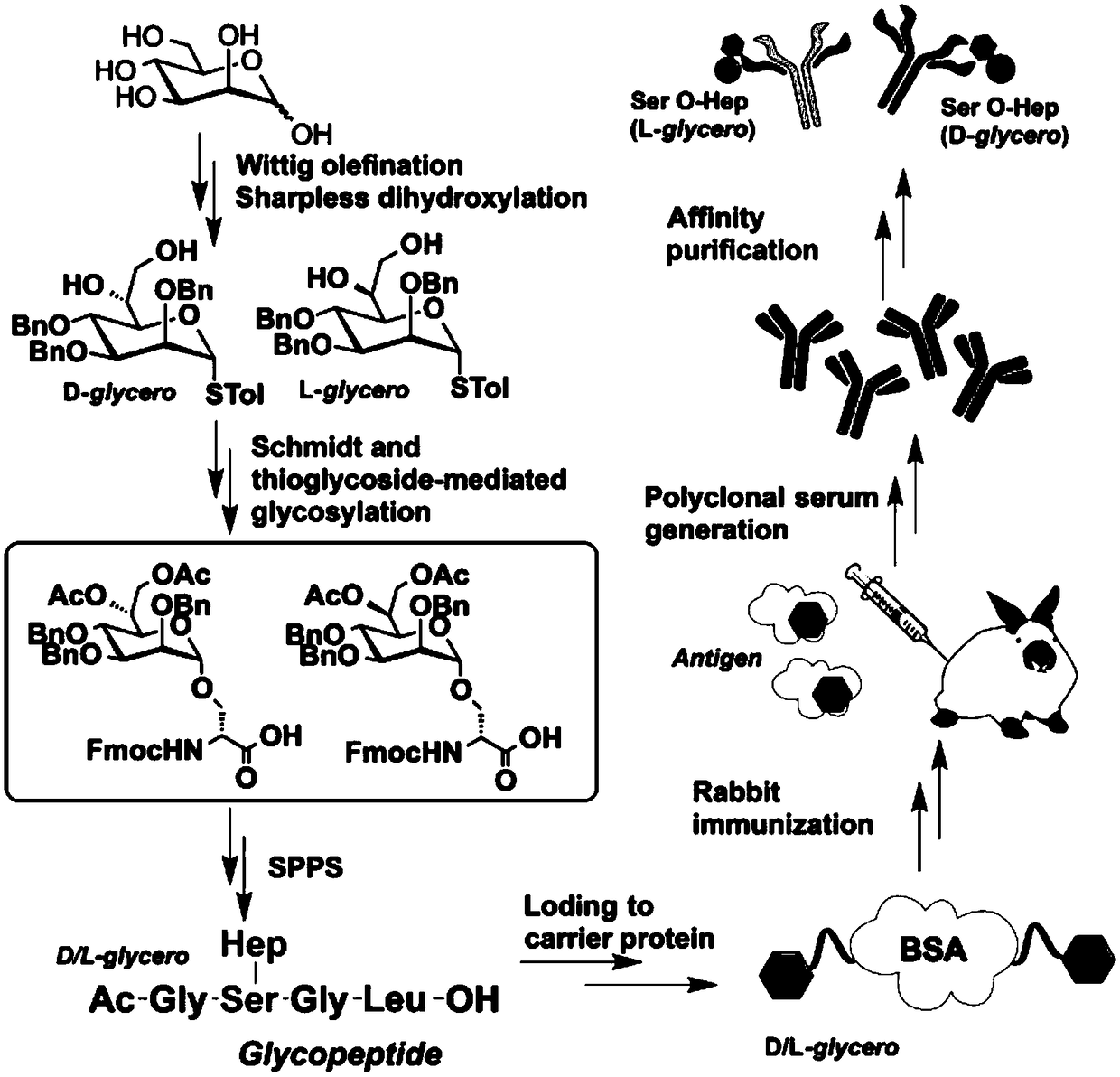
Polyclonal antibody for efficiently recognizing proteinserine heptanose glycosylation, and reparation method and application thereof
A technology of serine heptose and purification method, which is applied to peptide preparation methods, chemical instruments and methods, antibodies, etc., can solve the problems of lack of relevant tools and effective means for detection, and achieve good application prospects, good specificity, and production low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The synthesis of embodiment 1 key intermediate 6
[0061] Using mannose 2 as a raw material, under the action of acetic anhydride and iodine, the fully acetyl-protected mannose 3 was obtained. Under the catalysis of boron trifluoride ether, the end of mannose 3 was coupled with p-cresylthiol to obtain compound 4 , followed by deprotection of the hydroxyacetyl group of compound 4 under the action of sodium methoxide, the 6-position primary alcoholic hydroxyl was selectively protected with TBDMS to obtain compound 5, and compound 5 was protected with benzyl to obtain compound 6. In order to selectively expose the 6-position primary alcohol hydroxyl group, the primary alcohol hydroxyl group was selectively removed under the conditions of acetic acid and TBAF, and the compound 7 was obtained by TBDMS protection. Compound 7 was oxidized to Aldehydes can be used to obtain compound 8, and compound 8 undergoes a Wittig reaction to convert the aldehyde group into aglycone 9, and...
Embodiment 2
[0062] The synthesis of embodiment 2 heptose amino acids 16a and 13b
[0063] The 6-position D-type heptose amino acid was synthesized based on the glycosylation reaction of trichloroimidate, and the 6-position L-type heptose amino acid was synthesized based on the glycosylation reaction of glucosinolate.
[0064] Specifically, the synthesis of the heptose amino acid intermediate 16a is as follows: After compound 10a removes the terminal p-cresyl thiol under the action of NBS, the three hydroxyl groups of 1, 6, and 7 are acetylated under the condition of acetic anhydride pyridine Compound 12a was obtained by protection at the position, and compound 12a was selectively removed under the catalysis of hydrazine acetate to protect the acetyl group of terminal hydroxyl to obtain compound 13a, and 13a was converted into activated trichloroimidate under the action of trichloroacetonitrile and DBU, Using the activated trichloroimidate as a sugar donor, under the action of triflate, th...
Embodiment 3
[0066] Synthesis of Example 3 Serine Heptosylated Polypeptides 1a and 1b
[0067] For the synthetic route of hapten 1a, 1b, see Figure 7 .
[0068] First, 2-chlorotrityl resin is used as a solid phase carrier, and Fmoc-Leu-OH, Fmoc-Gly-OH, and 16aFmoc-Gly-OH are connected to the carrier in turn, and the terminal amino group is acetylated under the action of acetic anhydride pyridine. Glycopeptides and protective groups were cut off from the resin with a gentle cutting reagent (acetic acid / TFE / DCM=1:1:8) during cutting to obtain fully protected glycopeptide 18a; with 2-chlorotrityl Base resin as a solid phase carrier, Fmoc-Leu-OH, Fmoc-Gly-OH, 13b, Fmoc-Gly-OH are connected to the carrier in sequence, the terminal amino group is capped with acetyl group under the action of acetic anhydride pyridine, when cutting The glycopeptide together with the protecting group was cleaved from the resin with a mild cleavage reagent (acetic acid / TFE / DCM=1:1:8) to obtain fully protected gly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com