Method for separating and preparing exosome
A technology of exosomes and liquids, applied in the biological field, can solve problems such as pollution, multiple impurities, residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: A method for the isolation and preparation of exosomes
[0051] 1. Materials
[0052] Peripheral blood: from discarded samples after clinical examination of patients
[0053] Bone marrow plasma: from discarded specimens after clinical examination of patients
[0054] Reagent XBP, Reagent XWP, membrane adsorption column exoEasy Midi Spin Columns, column Spin Column comes from the kit QIAGEN exoRNeasy Serum / PlasmaMidi Kits catNo: 77044.
[0055] Reagent XE: QIAGEN Lot No.160011905
[0056] Exosome RNA extraction kit: exoRNeasy Serum / Plasma Midi Kit, Qiagen, Germany, cat.No.77044
[0057] 2. Method
[0058] 1. Centrifuge to remove cellular impurities: Centrifuge peripheral blood or bone marrow plasma at 4°C and 2500g for 10 minutes to remove the influence of cellular impurities such as red blood cells and white blood cells, discard the precipitate and retain the supernatant, and transfer 500 μl of the supernatant to a new 1.5 ml EP tube.
[0059] 2. C...
Embodiment 2
[0069] Example 2: Extraction of exosome total RNA
[0070] 1. Materials
[0071] Example 1 Obtaining a liquid sample containing exosomes
[0072] Exosome RNA extraction kit: exoRNeasy Serum / Plasma Midi Kit, Qiagen, Germany, cat.No.77044
[0073] 2. Method
[0074] 1. Add 700 μl QIAzol to the exosome-containing resuspension obtained above
[0075] 2. Vortex the EP tube for 5 seconds, let stand at room temperature (15°C-25°C) for 5 minutes, add 90 μl chloroform and shake vigorously up and down for 15 seconds, and let stand at room temperature for 2-3 minutes;
[0076] 3. Centrifuge at 12000g for 15min at 4°C;
[0077] 4. Transfer the upper aqueous phase to a new EP tube to avoid sucking into the middle organic layer;
[0078] 5. Add twice the volume of 100% ethanol to the upper layer of water (for example, add 800 μl of 100% ethanol to the upper layer of 400 μl of water), mix well by blowing up and down, draw 700 μl of liquid and add it to the column (RNeasy MinElute Spin C...
Embodiment 3
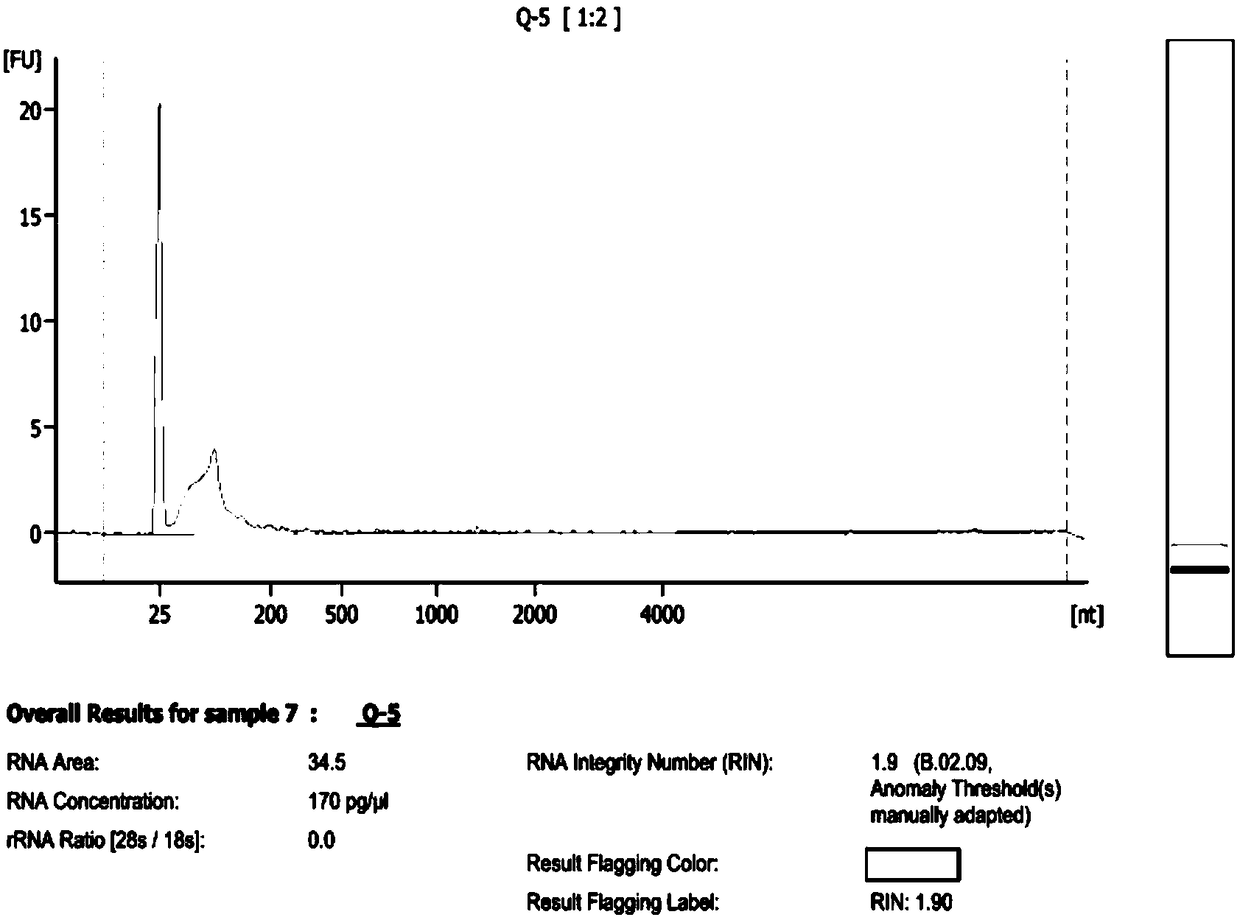
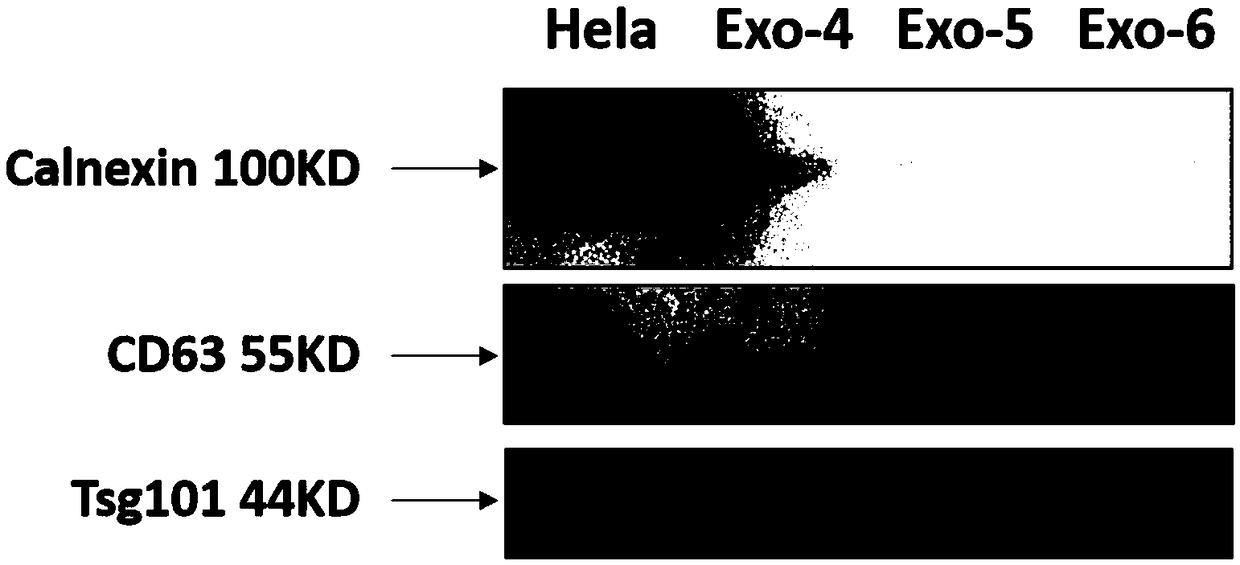
[0087] Example 3. Detection of RNA quality in exosomes and exosomes
[0088] 1. Materials
[0089] The exosome sample obtained in Example 1 (the sample number is Exo-6)
[0090] Exosome RNA obtained in Example 2 (sample number is sample7: Q-5)
[0091] CD63, TSG101, Calnexin (purchased from Abcam)
[0092] Marker: purchased from Thermo Scientific (#26617)
[0093] PVDF membrane: purchased from Immobilon-P (Cat No.IPVH00010)
[0094] Primary antibody diluent: purchased from Beyotime (P0023A)
[0095] HELA: Provided by the Institute of Basic Research, Chinese Academy of Medical Sciences
[0096] TBST: It is configured by 10×TBS plus 0.1% Tween-20
[0097] 10×TBS buffer:
[0098] Tris Base 24.2g
[0099] NaCl 80g
[0100] wxya 2 O 1000ml
[0101] TBST buffer:
[0102] 10×TBS 100ml
[0103] wxya 2 O 900ml
[0104] Tween-20 1ml
[0105] 2. Method -
[0106] (1) RNA quality inspection
[0107] BGI was entrusted to use the Agilent 2100 bioanalyzer for RNA quality in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com