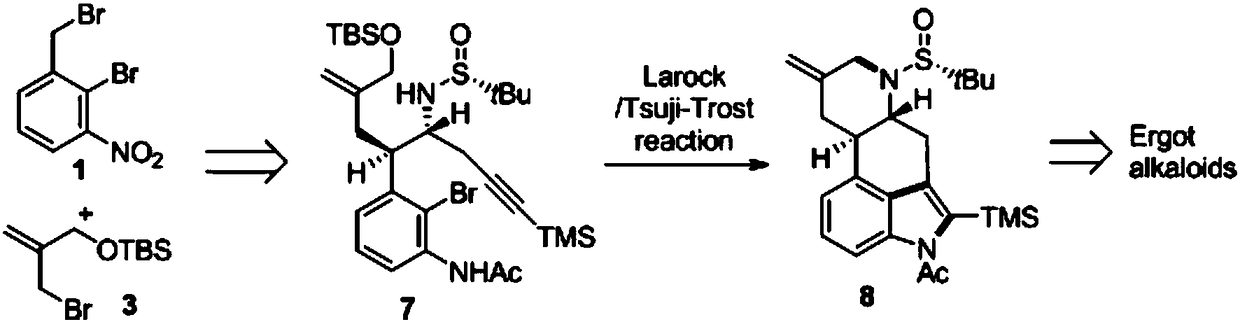
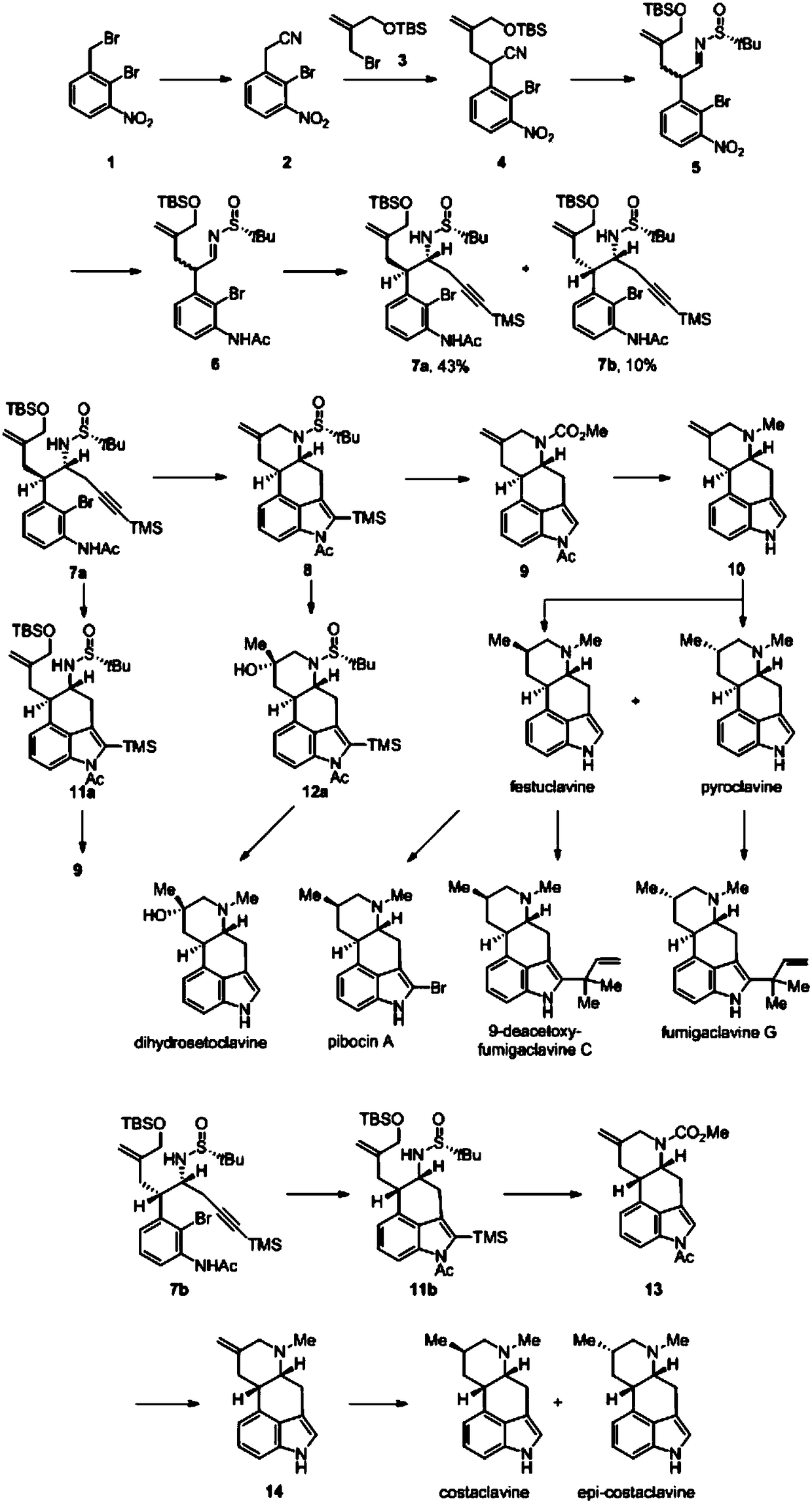
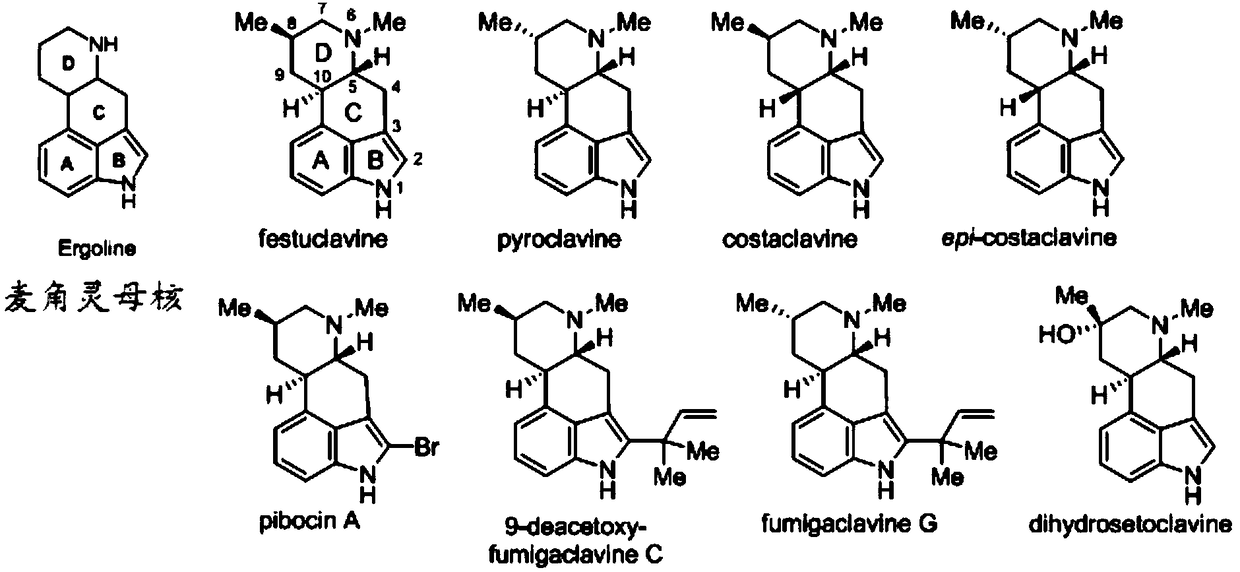
Asymmetric synthesis method for ergot alkaloids
A synthetic method and alkaloid technology, applied in the direction of asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., can solve the problems of long steps, difficulty in realizing industrial production, and no step-by-step construction of B/C/D rings, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 compound 2
[0063] Compound 1 was dissolved in a mixed solution of ethanol and water (the ratio of absolute ethanol to water was 5:1), potassium cyanide was added, and the molar ratio of compound 1 to potassium cyanide was 1:1.1. Stir at 25°C for 4h , ethanol was evaporated under reduced pressure, the remaining mixture was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, evaporated to dryness under reduced pressure, and the crude product was subjected to column chromatography (PE-EtOAc, 3:1). Compound 2 was isolated as a yellow solid.
[0064] The yield of this step is 75%, and the relevant analysis data are as follows:
[0065] 1 H NMR (400MHz, CDCl 3 )δ7.79(d, J=7.6Hz, 1H), 7.74(d, J=8.0Hz, 1H), 7.45(t, J=8.0Hz, 1H), 4.66(s, 2H); 13 C NMR (100MHz, CDCl 3 ) δ 133.2, 132.4, 128.7, 124.8, 115.9, 115.3, 25.5.
Embodiment 2
[0066] The preparation of embodiment 2 compound 4
[0067] Compound 2 was dissolved in 0.3M tetrahydrofuran (THF) solution, cooled to -78°C, lithium hexamethyldisilazide was added dropwise, and after stirring for 10 minutes, the THF solution of compound 3 was added dropwise to the reaction solution, dropwise After the addition was complete, the temperature was raised to 25° C., and the reaction was stirred for 4 h, then quenched by adding a saturated NaCl solution, and extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure. The crude product was separated by column chromatography (PE-EtOAc, 6:1) to obtain compound 4 as a pale yellow oil. The molar ratio of compounds 2 and 3 to lithium hexamethyldisilazide is 1:1.1:1.1.
[0068] The yield of this step is 90%, and the relevant analysis data are as follows
[0069] 1 H NMR (400MHz, CDCl 3 )δ7.8...
Embodiment 3
[0070] The preparation of embodiment 3 compound 5
[0071] Compound 4 was dissolved in 0.2M toluene solution, the temperature was lowered to -78°C, and diisobutylaluminum hydride was added dropwise according to the molar ratio of compound 4 and diisobutylaluminum hydride being 1:1.1. React at -78°C for 30 minutes, slowly add saturated sodium bicarbonate solution dropwise at 25°C until the solid precipitates, filter with suction, wash the solid with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and evaporate to dryness under reduced pressure to obtain The crude product (aldehyde compound) was directly sent to the next step without purification.
[0072] The crude product and (R)-tert-butylsulfinamide were dissolved in 0.36M tetrahydrofuran solution, and tetraethoxytitanium (Ti(OEt) was added 4 ), the molar ratio of the crude product to tert-butylsulfinamide and tetraethoxytitanium is 1:2:2. React at 25°C for 6h, add saturated sodium chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com