Method for preparing perovskite-type catalytic material
A catalytic material, perovskite-type technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of complex preparation process, low surface area, surface catalytic reaction efficiency, and high cost. To achieve the effects of inhibiting particle growth, improving performance, and improving particle agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment provides a method for preparing a perovskite-type catalytic material, which includes the following steps,
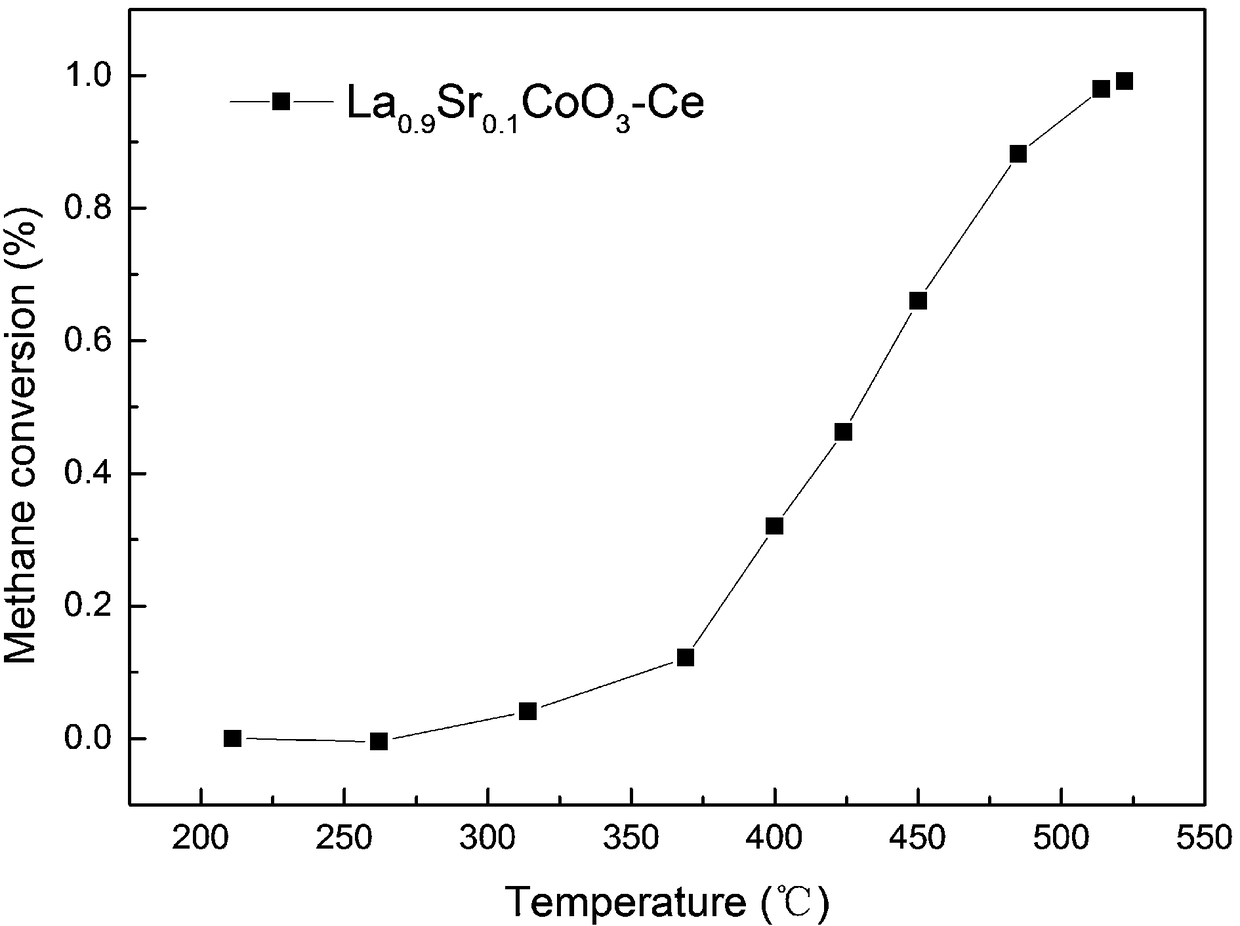
[0030] (1) Weigh the perovskite metal oxide and metal nitrate precursor respectively; the perovskite metal oxide is LaCoO 3 , LaMnO 3 、LaFeO 3 、LaNiO 3 、LaCrO 3 , La 1-x Sr x CoO 3 、LaCo 1-x Mg x o 3 or La 1-x Ce x CoO 3 One of. Present embodiment 1 selects La for use 0.9 Sr 0.1 CoO 3 .
[0031] The metal nitrate precursor is one of rare earth nitrates, transition metal nitrates or alkaline earth metal nitrates. The rare earth nitrate is one of lanthanum nitrate, cerium nitrate, gadolinium nitrate, samarium nitrate or neodymium nitrate. The transition metal nitrate is one of cobalt nitrate, manganese nitrate, iron nitrate, nickel nitrate, copper nitrate or chromium nitrate. The alkaline earth metal nitrate is one of magnesium nitrate, calcium nitrate, strontium nitrate or calcium nitrate.
[0032] In this embodiment, the meta...
Embodiment 2
[0042] This embodiment provides a method for preparing a perovskite-type catalytic material, which includes the following steps,
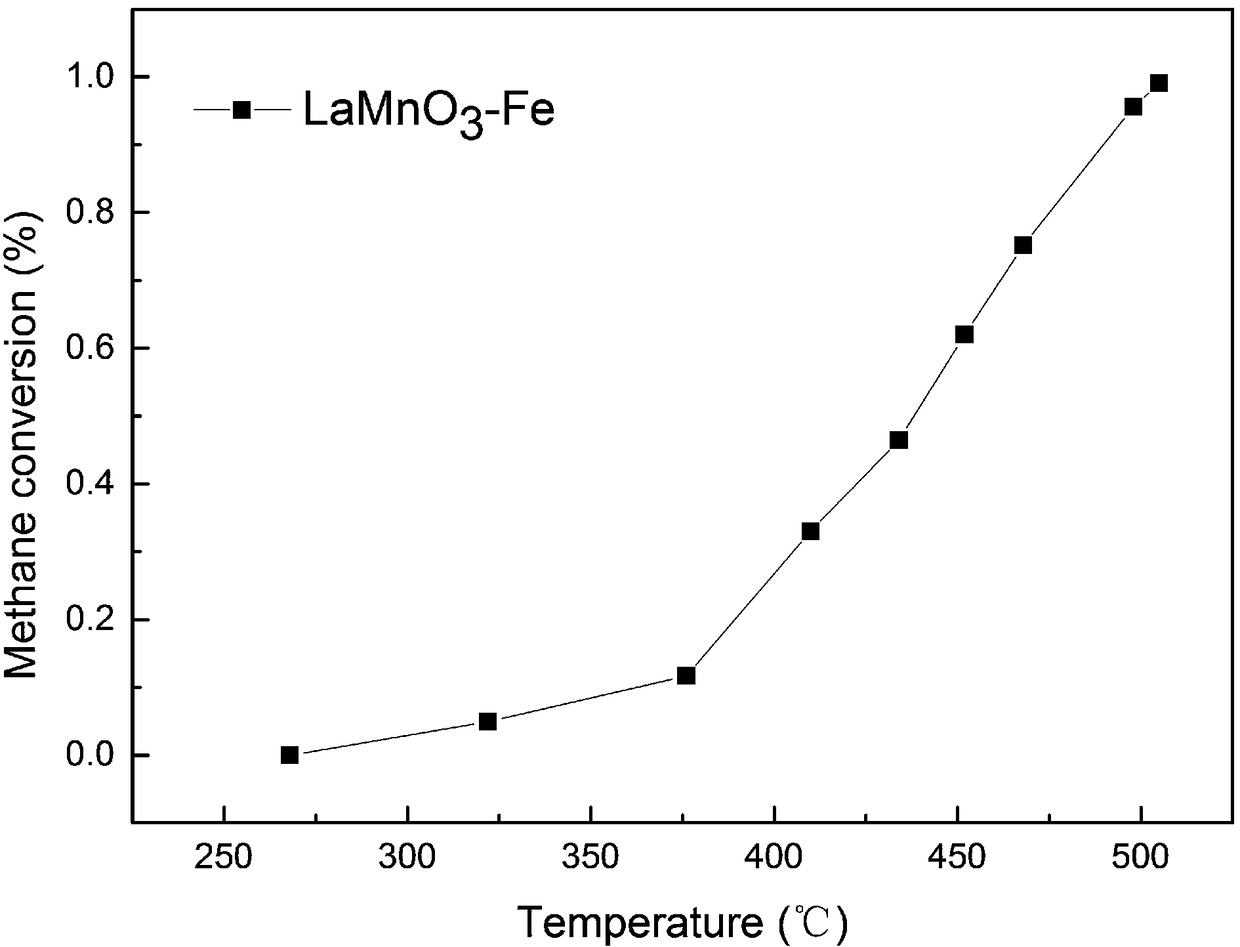
[0043] (1) Weigh the perovskite metal oxide and metal nitrate precursor respectively; the perovskite metal oxide is LaCoO 3 , LaMnO 3 、LaFeO 3 、LaNiO 3 、LaCrO 3 , La 1-x Sr x CoO 3 、LaCo 1-x Mg x o 3 or La 1-x Ce x CoO 3 One of. This embodiment chooses LaMnO 3 .
[0044] The metal nitrate precursor is one of rare earth nitrates, transition metal nitrates or alkaline earth metal nitrates. The rare earth nitrate is one of lanthanum nitrate, cerium nitrate, gadolinium nitrate, samarium nitrate or neodymium nitrate. The transition metal nitrate is one of cobalt nitrate, manganese nitrate, iron nitrate, nickel nitrate, copper nitrate or chromium nitrate. The alkaline earth metal nitrate is one of magnesium nitrate, calcium nitrate, strontium nitrate and calcium nitrate.
[0045] In this embodiment, the metal nitrate precursor is tra...
Embodiment 3
[0053] This embodiment provides a method for preparing a perovskite-type catalytic material, which includes the following steps,
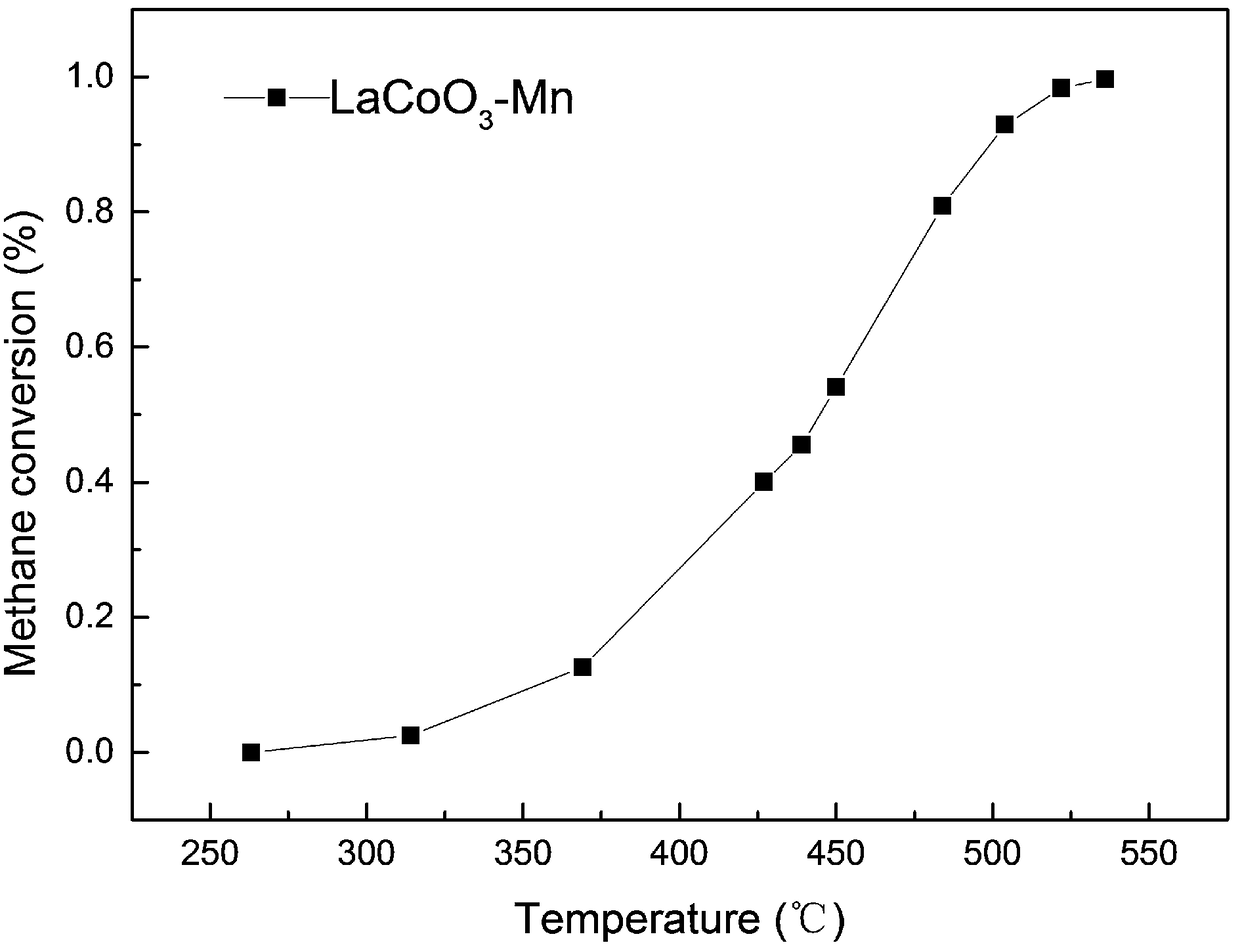
[0054] (1) Weigh the perovskite metal oxide and metal nitrate precursor respectively; the perovskite metal oxide is LaCoO 3 .
[0055] The metal nitrate precursor is one of rare earth nitrates, transition metal nitrates or alkaline earth metal nitrates. The rare earth nitrate is one of lanthanum nitrate, cerium nitrate, gadolinium nitrate, samarium nitrate or neodymium nitrate. The transition metal nitrate is one of cobalt nitrate, manganese nitrate, iron nitrate, nickel nitrate, copper nitrate or chromium nitrate. The alkaline earth metal nitrate is one of magnesium nitrate, calcium nitrate, strontium nitrate and calcium nitrate.
[0056] In this embodiment, the metal nitrate precursor is transition metal nitrate Mn(NO 3 ) 2 ;
[0057] (2) Put the perovskite metal oxide, metal nitrate precursor and deionized water in step (1) into the reacto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com