Method for synthesizing chlorodifluoroethane from dichlorodifluoroethane
A technology of difluorodichloroethane and difluoromonochloroethane, which is applied in the field of fluorine chemical industry, can solve problems such as toxicity, destruction of atmospheric ozone layer, unfavorable industrial production, etc., and achieves high selectivity and conversion rate, high conversion rate, The effect of solving pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
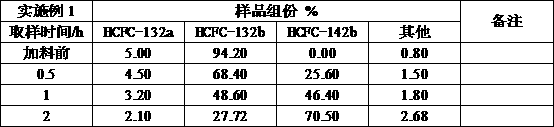
Embodiment 1
[0025] Add HCFC-132b with a purity of 94%, add 120ml of HCFC-132b, add 200ml of saturated calcium hydroxide anhydrous ethanol solution, use a 1L reactor with stirring, control the reaction temperature at 75°C, and the reaction pressure at 0.4-0.8Mpa Between, the reaction speed is controlled at 300-600r / min, after 3 hours of reaction, sampling analysis shows that the conversion rate of the final product HCFC-142b is 75%, and the selectivity is 98%.
[0026]
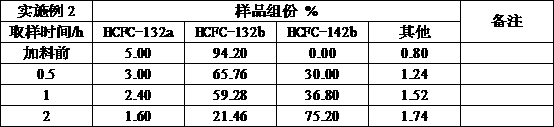
Embodiment 2
[0028] Add HCFC-132b with a purity of 94%, add 150ml of HCFC-132b, add 200ml of saturated calcium hydroxide anhydrous ethanol solution, use a 1L reactor with stirring, control the reaction temperature at 120°C, and the reaction pressure at 0.6-1.2Mpa Between, the reaction speed is controlled at 300-600r / min, after 4 hours of reaction, sampling analysis shows that the conversion rate of the final product HCFC-142b is 80%, and the selectivity is 99%.
[0029]
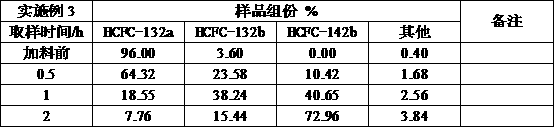
Embodiment 3
[0031] Add HCFC-132a with a purity of 96%, add 150ml of HCFC-132a, add 200ml of saturated sodium hydroxide anhydrous ethanol solution, use a 1L reactor with stirring, control the reaction temperature at 90°C, and the reaction pressure at 0.5-1.0Mpa Between, the reaction speed is controlled at 300 ~ 600r / min, after 4 hours of reaction, sampling analysis shows that the conversion rate of the final product HCFC-142b is 76%, and the selectivity is 96%.
[0032]
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical temperature | aaaaa | aaaaa |
| critical pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com