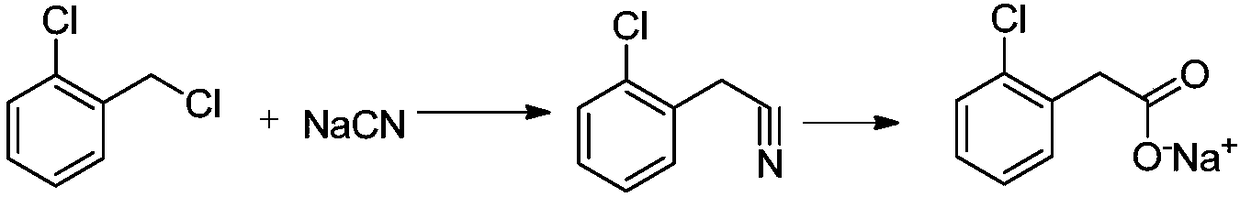
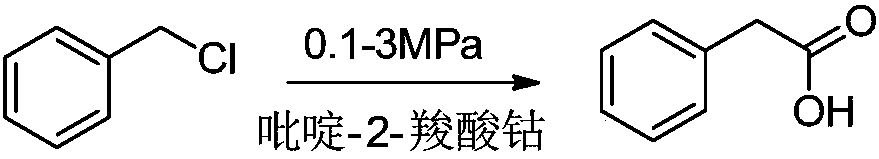
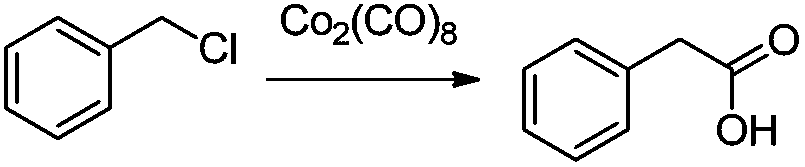Preparation method of phenylacetic acid type compound
The technology of a compound and phenylacetic acid, applied in the field of preparation of phenylacetic acid compounds, can solve the problems of large amount of catalyst, toxic three wastes, complicated operation, etc., and achieve the effects of reducing the overall usage amount, reducing the reaction pressure, and being easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Add 64.4g (0.4mol) of p-chlorobenzyl chloride, add 0.55g of cobalt pyridine-2-carboxylate, 1.37g of palladium acetate, add 160g (1.2mol) of 30% NaOH, 73.7g of methanol (1.6mol) and replace the system with CO three times , heated up to 150°C, charged with CO until the pressure in the kettle was 1.5MPa, kept the temperature for 5hrs, cooled to room temperature, distilled under reduced pressure to remove methanol, filtered off the catalyst, added 30% hydrochloric acid to adjust the pH to 1, filtered, and dried to obtain p-chloro Phenylacetic acid, the purity is: 99.76%, the yield is 85%.
Embodiment 2
[0042]Add 64.4g (0.4mol) of p-chlorobenzyl chloride, add 0.55g of cobalt pyridine-2-carboxylate, 1.37g of palladium acetate, add 121g (1.2mol) of triethylamine, 73.7g of methanol (1.6mol) and replace the system with CO 3 times , heat up to 150°C, pour in CO until the pressure in the kettle is 1.5MPa, keep the temperature for 5hrs, cool down to room temperature, remove methanol and part of triethylamine by distillation under reduced pressure, filter out the catalyst, add 30% hydrochloric acid to adjust the pH to 1, filter , drying to obtain p-chlorophenylacetic acid with a purity of 99.25% and a yield of 80%.
Embodiment 3
[0044] Add 64.4g (0.4mol) of p-chlorobenzyl chloride, add 0.773g of cobalt pyridine-2-carboxylate, 1.16g of palladium acetate, add 160g (1.2mol) of 30% NaOH, 73.7g of methanol (1.6mol) and replace the system with CO three times , heated up to 150°C, charged with CO until the pressure in the kettle was 1.5MPa, kept the temperature for 5hrs, cooled to room temperature, distilled under reduced pressure to remove methanol, filtered off the catalyst, added 30% hydrochloric acid to adjust the pH to 1, filtered, and dried to obtain p-chloro Phenylacetic acid, the purity is: 99.29%, the yield is 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com