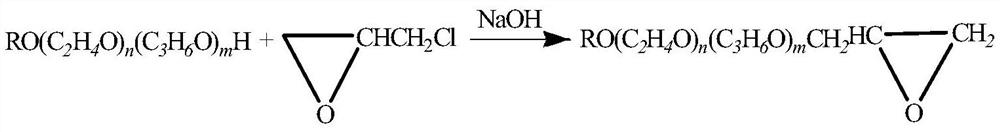
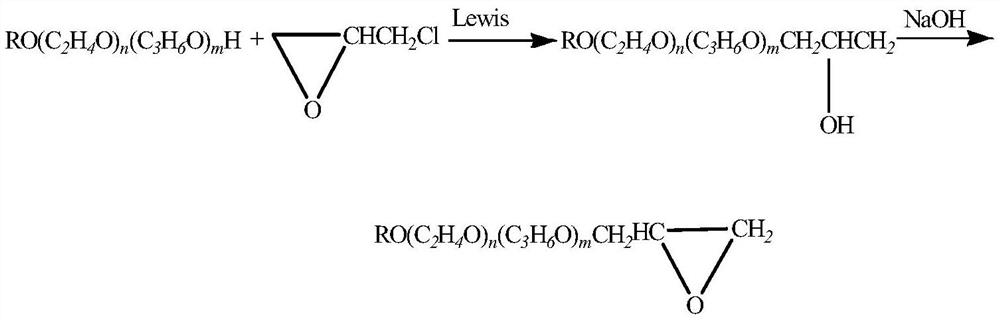
A kind of synthetic method of epoxy-terminated polyether
A technology of end-capping polyether and synthesis method, which is applied in the field of synthesis of epoxy-terminated polyether, and can solve problems such as inconvenient use, poor selectivity, and insufficient epoxy end-capping rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 166g of ZnCl 2 Dissolve in 1000ml of distilled water, add 6mol / l NaOH solution drop by drop to form a white precipitate, wash the precipitate until there is no chloride ion, then add 1mol / l HClO dropwise 4 Solution, until the white precipitate is basically dissolved, filter, remove a small amount of insoluble matter, obtain a colorless liquid, transfer it to an evaporating dish, heat until white crystals appear, and dry at 70 ° C in an oven to obtain 261g of Zn(ClO 4 ) 2 catalyst.
Embodiment 2
[0034] Weigh 133g of AlCl 3 Dissolve in 1000ml of distilled water, add 6mol / l NaOH solution drop by drop to form a white precipitate, wash the precipitate until there is no chloride ion, then add 1mol / l HClO dropwise 4 Solution, until the white precipitate is basically dissolved, filter, remove a small amount of insoluble matter, obtain a colorless liquid, transfer to an evaporating dish, heat until white crystals appear, and dry in an oven at 70°C to obtain 305g of Al(ClO 4 ) 3 catalyst.
Embodiment 3
[0036] Add 1000 g of methoxypolyether (molecular weight 200, n≈4) and 3 g of the catalyst of Example 1 into a 5 L reaction bottle, turn on the stirrer, stir for 1 h, and then move it into metering tank 1, and the 472 g ring that was drawn into metering tank 2 in advance Oxychloropropane was continuously fed into the microchannel reactor (at a constant temperature of 40° C.) by a plunger pump to react in proportion. The reaction time in the microchannel reactor was 5 seconds, and the reaction solution was continuously collected to obtain semi-finished α-chlorohydrins.
[0037] Transfer the collected α-chlorohydrin semi-finished product into a closed-loop reactor, start stirring, cool down to room temperature, add 200g of solid NaOH, react at 35-40°C for 3 hours, cool down to room temperature, and obtain a crude product.
[0038] After the crude product is filtered to desalt, the filtrate is collected and transferred to a refining reactor, then phosphoric acid is added to neutral...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com