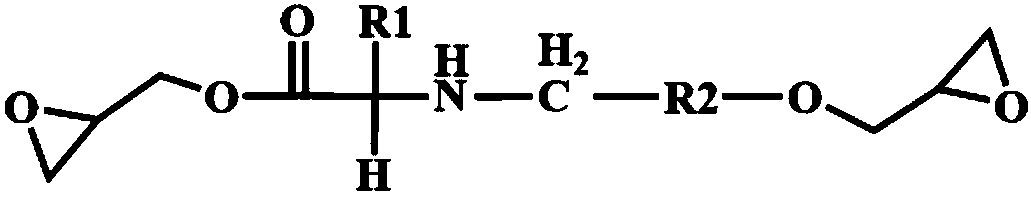Bio-based epoxy resin based on natural amino acids, and preparation method and application thereof
A technology of natural amino acid and epoxy resin, applied in the direction of organic chemistry, etc., to achieve the effect of saving petroleum resources, good controllability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 1 mol of alanine, 1.2 mol of vanillin, and 1 mol of KOH in 250 mL of ethanol, react at 30°C for 24 hours, add 3 mol of sodium borohydride and control the reaction temperature at 10°C, at this temperature The reaction was continued for 36 hours to finally obtain the alanine-vanillin multifunctional monomer with a yield of 89%.
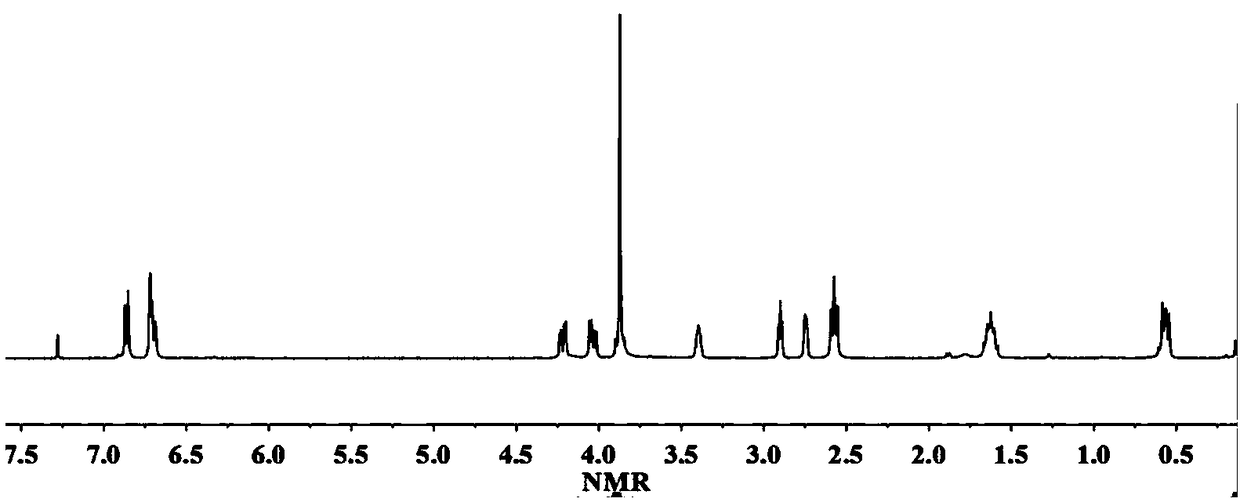
[0039] React 1 mol of alanine-vanillin multifunctional monomer and 5 mol of epichlorohydrin in the presence of 0.1 mol of tetrabutylammonium bromide at 100°C for 2 hours, then remove the solvent by rotary evaporation under reduced pressure, wash with water And after drying, the epoxidized alanine-vanillin compound was obtained with a yield of 93%. H NMR spectrum 1 H-NMR such as figure 1 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the epoxidized alanine-vanillin dicyanide compound structure.
[0040]Mix the obtained epoxidized alanine-vanillin compound and curing agent DDS in acetone acc...
Embodiment 2
[0042] Dissolve 1 mol of lysine, 2.5 mol of vanillin, and 1.1 mol of KOH in 200 mL of ethanol, react at 50°C for 16 hours, add 4 mol of sodium borohydride and control the reaction temperature at 20°C, at this temperature The reaction was continued for 24 hours to finally obtain a lysine-vanillin multifunctional monomer with a yield of 81%.
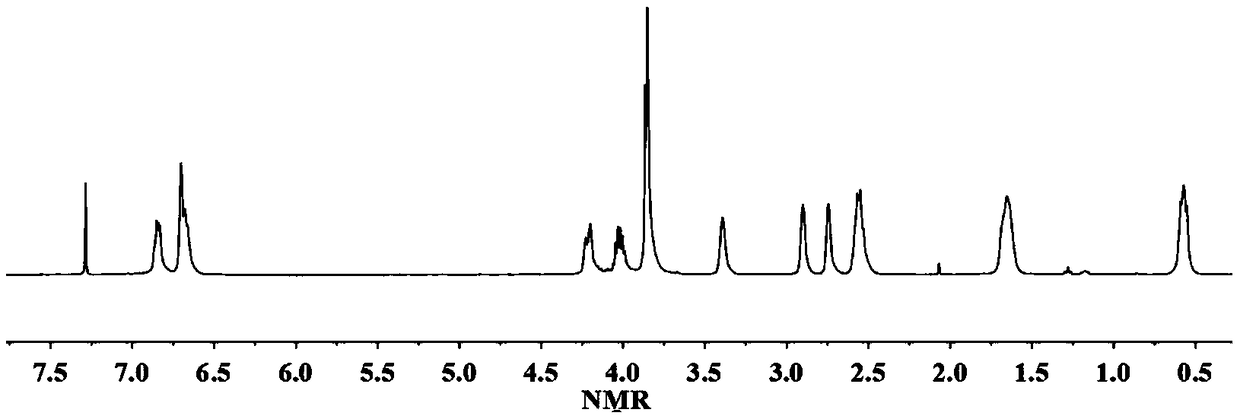
[0043] React 1mol lysine-vanillin multifunctional monomer and 8mol epichlorohydrin in the presence of 0.15mol tetrabutylammonium bromide at 80°C for 5 hours, then remove the solvent by rotary evaporation under reduced pressure, wash with water And after drying, the epoxidized lysine-vanillin compound was obtained with a yield of 91%. H NMR spectrum 1 H-NMR such as figure 2 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the epoxidized lysine-vanillin dicyanide compound structure.
[0044] Mix the obtained epoxidized lysine-vanillin compound and the curing agent DDS in acetone according to th...
Embodiment 3
[0046] Dissolve 1 mol of tyrosine, 1.5 mol of vanillin, and 1.5 mol of KOH in 180 mL of ethanol, react at 80°C for 12 hours, add 2 mol of sodium borohydride and control the reaction temperature at 30°C, at this temperature The reaction was continued for 12 hours to finally obtain a tyrosine-vanillin multifunctional monomer with a yield of 79%.
[0047] React 1mol tyrosine-vanillin multifunctional monomer and 12mol epichlorohydrin in the presence of 0.2mol tetrabutylammonium bromide at 70°C for 8 hours, then remove the solvent by rotary evaporation under reduced pressure, wash with water And after drying, the epoxidized tyrosine-vanillin compound was obtained with a yield of 87%.
[0048] Mix the obtained epoxidized tyrosine-vanillin compound and the curing agent DDS in acetone according to the molar ratio of epoxy and NH one to one, then heat and pre-cure in a blast oven, and finally carry out at 180°C After curing for 2 hours, a cured product of tyrosine-vanillin-DDS epoxy r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com