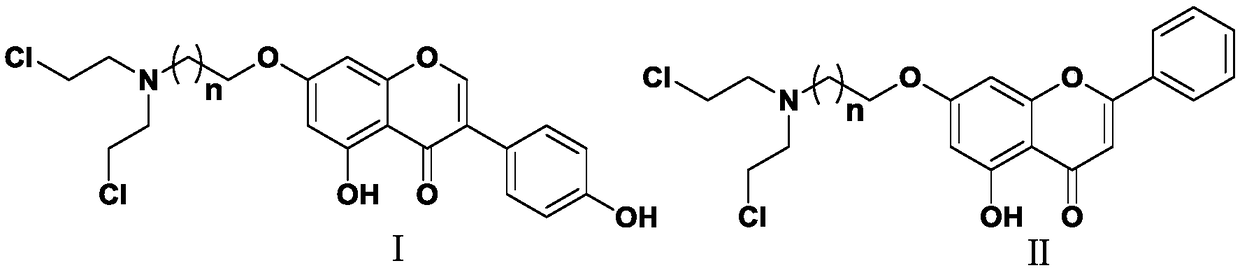
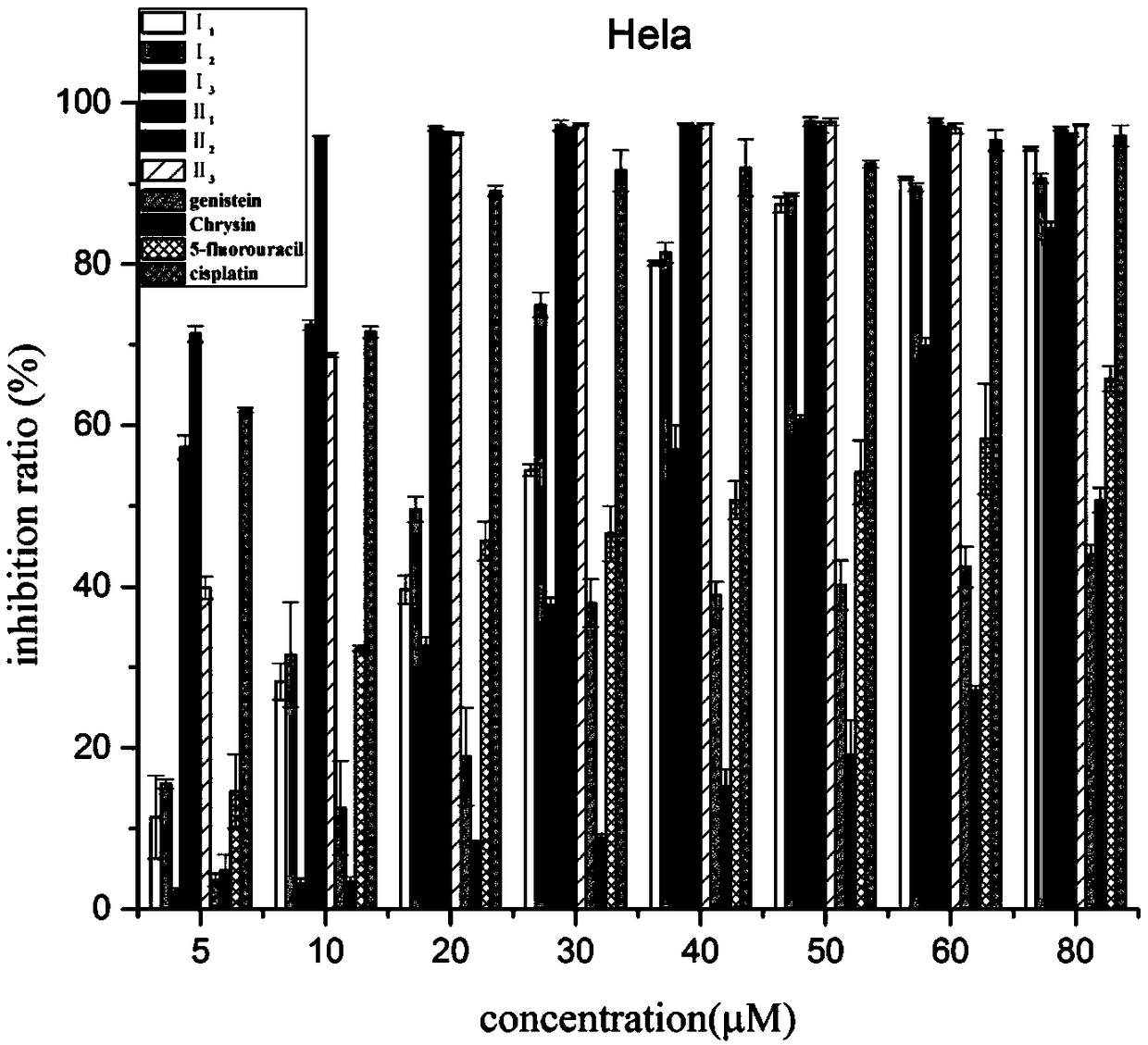
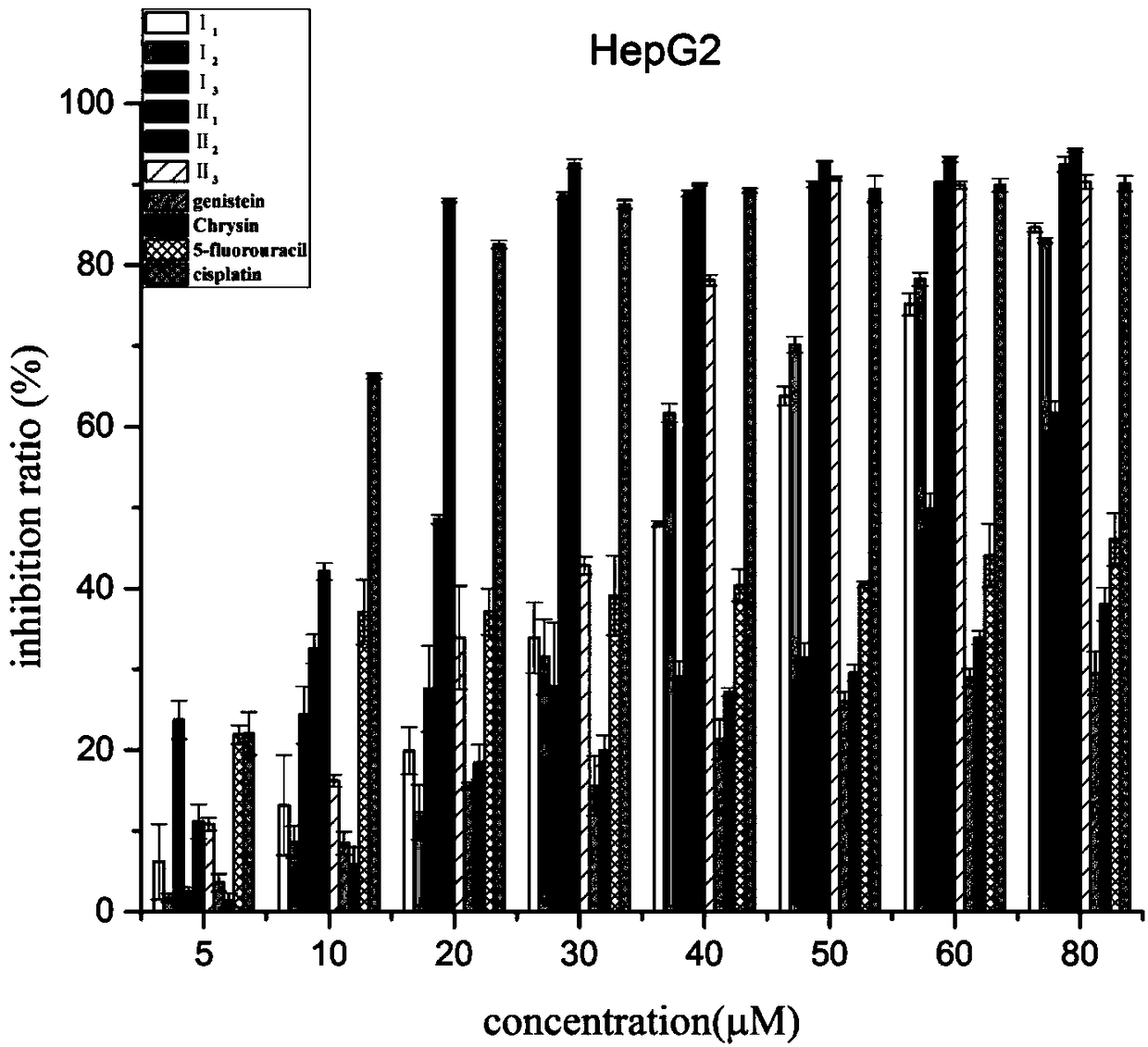
Nitrogen mustard-based flavonoid derivative, preparation method thereof and application in anti-tumor direction
A mustard-based flavonoid and derivative technology, applied in the field of medicinal chemistry, can solve the problems of low bioavailability, poor fat solubility and water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention also provides a preparation method of nitrogen-containing mustardyl flavonoid derivatives, comprising:
[0041] S1) reacting genistein or chrysin with a halogenated alkane represented by formula III under alkaline conditions to obtain compounds represented by formula IV and formula V;
[0042]
[0043] S2) reacting the compounds represented by formula IV and V with diethanolamine to obtain compounds represented by formula VI and VII;
[0044]
[0045] S3) reacting compounds represented by formulas VI and VII with thionyl chloride to obtain nitrogen-containing mustardyl flavonoid derivatives represented by formulas I and II;
[0046]
[0047] Wherein, X is a halogen; n is an integer greater than or equal to 0, preferably an integer of 1-3.
[0048] React genistein with the halogenated alkane represented by formula III under alkaline conditions; the molar ratio of the genistein to the halogenated alkane represented by formula III is prefera...
Embodiment 1
[0064] Target compound Ⅰ 1 preparation of
[0065] step 1:
[0066] Genistein (2.7g, 10mmol), anhydrous K 2 CO 3 (2.76g, 20mmol), 10mL of 1,2-dibromoethane and 150mL of acetone were added to a 250mL round bottom flask, heated and stirred at reflux for about 8h. Then stop the reaction, remove acetone by rotary evaporation, add petroleum ether to wash the obtained mixture to remove 1,2-dibromoethane, filter under reduced pressure, separate solid from liquid, and collect the solid. Wash with a large amount of distilled water to remove potassium carbonate, and dry. The crude product adopts the method purification of column chromatography (eluent consists of: CH 2 Cl 2 :CH 3 OH=60:1), the yellow solid 2a was finally obtained with a yield of 70%. 1 H NMR (400MHz, DMSO-D6) δ12.96(s, 1H), 9.58(s, 1H), 8.41(s, 1H), 7.38(d, J=8.6Hz, 2H), 6.82(d, J= 8.7Hz, 2H), 6.69(d, J=2.3Hz, 1H), 6.43(d, J=2.3Hz, 1H), 4.47–4.43(m, 2H), 3.86–3.78(m, 2H).
[0067] Step 2:
[0068] The above-p...
Embodiment 2
[0072] Target compound Ⅰ 2 preparation of
[0073] step 1:
[0074] Genistein (2.7g, 10mmol), anhydrous K 2 CO 3 (2.76g, 20mmol), 10mL 1,3-dibromopropane and 150mL acetone were added to a 250mL round bottom flask, and the rest of the steps were the same as step 1 in Example 1. A yellow solid 2b was obtained in 69% yield. 1 H NMR (600MHz, DMSO-D6) δ9.59(s, 1H), 8.41(s, 1H), 7.38(d, J=8.6Hz, 2H), 6.81(d, J=8.6Hz, 2H), 6.68 (d, J=2.2Hz, 1H), 6.42(d, J=2.2Hz, 1H), 4.20(t, J=6.0Hz, 2H), 3.66(t, J=6.6Hz, 2H), 2.26(p ,J=6.3Hz,2H).
[0075] Step 2:
[0076] The intermediate product 2b (0.391 g, 1 mmol) prepared above, diethanolamine (1.05 g, 10 mmol), and 50 mL of acetonitrile were added to a 100 mL round bottom flask, and the rest of the steps were the same as step 2 in Example 1. 3b was obtained as a pale yellow solid in 85% yield. 1 H NMR (600MHz, METHANOL-D4) δ8.09(s, 1H), 7.38(d, J=8.4Hz, 2H), 6.85(d, J=8.5Hz, 2H), 6.52(s, 1H), 6.35 (s,1H),4.14(t,J=6.0Hz,2H),3.62(t,J=5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com