Compound with PD-L1 (programmed death-ligand 1) inhibitory activity as well as preparation method and application of compound
A compound, C1-C4 technology, applied in the direction of organic active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve the problems of easy immunogenicity, high production cost, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0144] The preparation of formula I compound
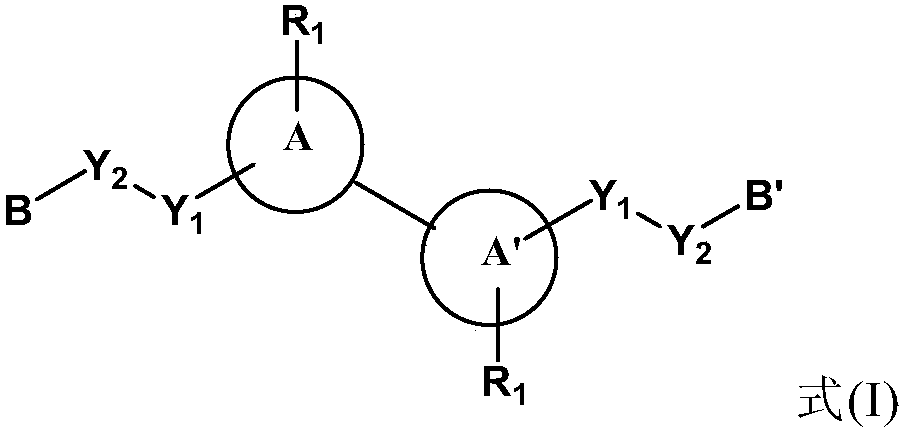
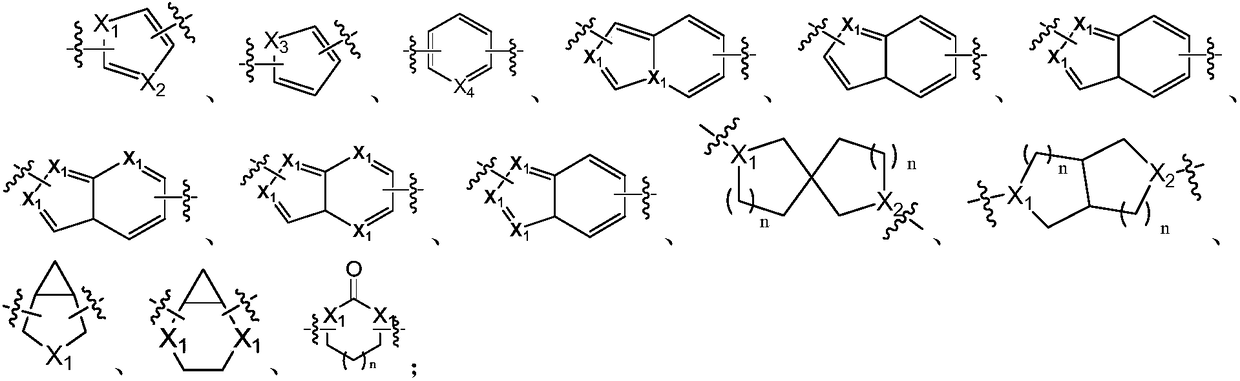
[0145]The following reaction schemes exemplify the preparation of compounds of formula I, their stereoisomers or mixtures thereof, or pharmaceutically acceptable salts thereof, wherein each group is as in the embodiment section of the compound of formula I above mentioned. It is to be understood that in the following reaction schemes, combinations of substituents and / or variables within the general formulae are permissible only if such combinations result in stable compounds. It should also be understood that other general formulas, such as general formula (Ia), (Ib), (Ic), (Id), and other formula I compounds specifically disclosed herein can be disclosed herein by those skilled in the art of organic chemistry ( Preparation is carried out by employing appropriately substituted starting materials and modifying the synthesis parameters as necessary using methods well known to those skilled in the art) or known methods.
[0146] Th...
Embodiment 1
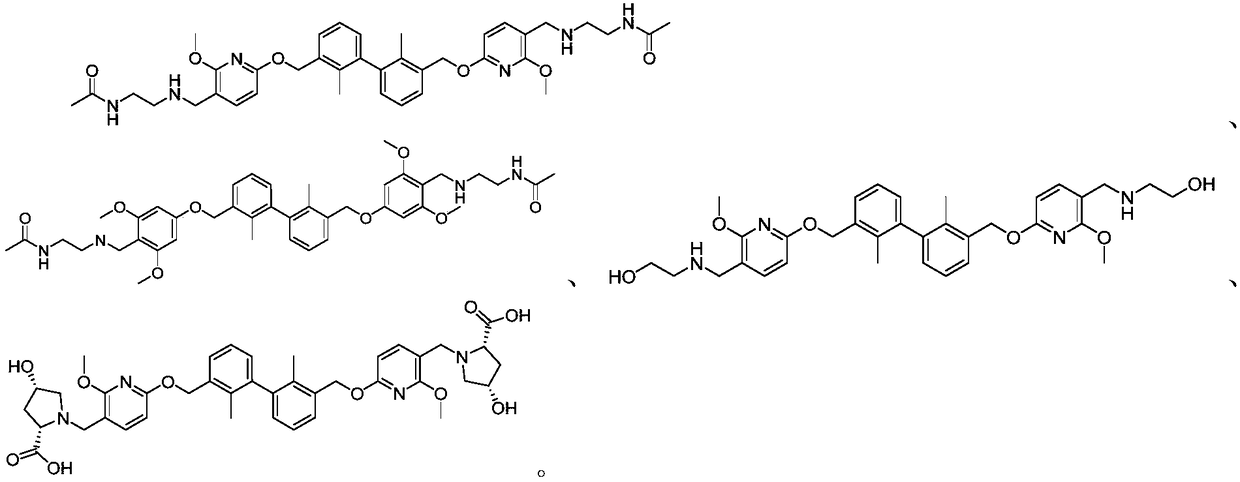
[0189] 2,2,2-Trifluoro-N-((6-((3'-(hydroxymethyl)-2,2'-dimethyl-[1,1'-biphenyl]-3-yl) Methoxy)-2-methoxypyridin-3-yl)methyl)acetamide
[0190]
[0191] tert-Butyl((6-((3'-(hydroxymethyl)-2,2'-dimethyl-[1,1'-biphenyl]-3-yl)methoxy)-2- Methoxypyridin-3-yl)methyl)carbamate
[0192]
[0193] To intermediate A (50 mg, 0.20 mmol), tert-butyl ((6-chloro-2-methoxypyridin-3-yl) methyl) carbamate (56 mg, 0.20 mmol), cesium carbonate (134 mg , 0.41mmol) in toluene (1mL) was added palladium acetate (4.6mg, 0.02mmol) and 2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl (17.5mg, 0.041mmol ), replaced with nitrogen for 3 minutes and heated and sealed at 110°C overnight. The reaction liquid was filtered with celite, the filtrate was concentrated, and the residue was separated and purified with a preparative plate (petroleum ether / ethyl acetate=4 / 1) to obtain the target compound (20 mg, 20%) as a yellow oil.
[0194] MS(ESI):m / z=479.3[M+H] + .
[0195] 1 H NMR (400MHz, DMSO-...
Embodiment 2
[0202] N-(2-(((6-((3'-(hydroxymethyl)-2,2'-dimethyl-[1,1'-biphenyl]-3-yl)methoxy)- 2-methoxypyridin-3-yl)methyl)amino)ethyl)acetamide
[0203]
[0204] N-(2-(((6-chloro-2-methoxypyridin-3-yl)methyl)amino)ethyl)acetamide
[0205]
[0206] 6-Chloro-2-methoxynicotinaldehyde (400mg, 2.34mmol) and N-(2-aminoethyl) acetamide (240mg, 2.34mmol) were mixed in ethanol (10mL), stirred at reflux for 2 hours and then reduced Concentrated under reduced pressure, the crude product was redissolved in methanol (10 mL), sodium borohydride (89 mg, 2.34 mmol) was added in portions, and the reaction was stirred at room temperature for 2 hours. After the solvent was removed by concentration under reduced pressure, the crude product was purified by silica gel column chromatography (dichloromethane / methanol 50 / 1-10 / 1 elution) to obtain compound 2A (350 mg, 58%).
[0207] 1 H NMR (400MHz, DMSO-d 6 )δ7.78(brS,1H),7.73(d,J=7.6Hz,1H),7.06(d,J=7.56Hz,1H),3.88(s,3H),3.61(s,2H),3.15- 3.10 (m, 2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com