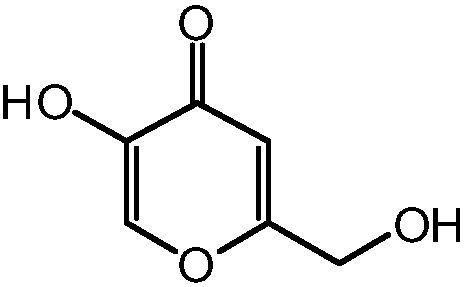
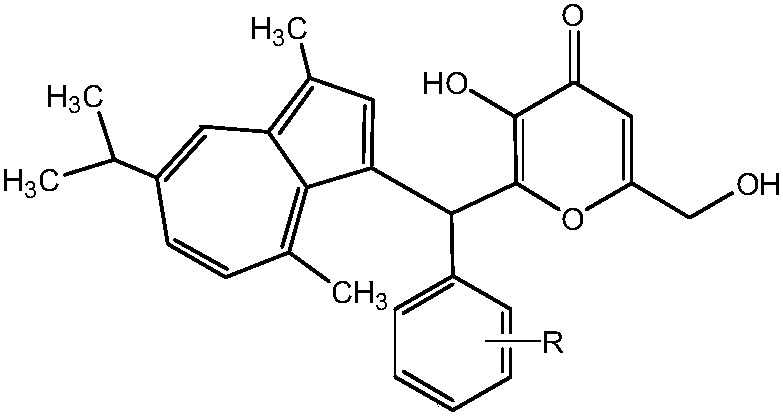
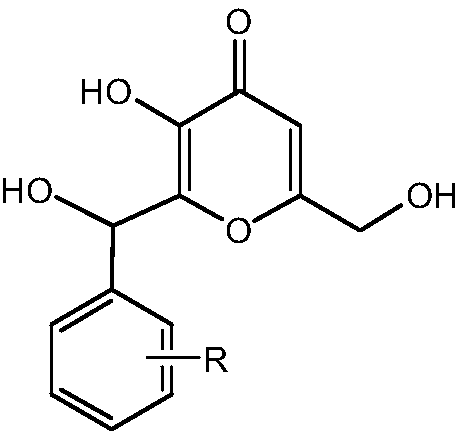
Kojic acid derivative containing guaiazulene structure and preparation method and application thereof
A technology of guaiazulene and kojic acid derivatives, which can be applied to medical preparations containing active ingredients, applications, and skin care preparations, etc., and can solve poor skin absorption, poor stability of kojic acid, and restrictions on kojic acid. problems such as the practical application of acid products, and achieve the effect of strong development and application prospects and strong inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 2-(3,8-Dimethyl-6-isopropylazulene-1-yl)phenylmethyl-3-hydroxy-6-hydroxymethyl-4H-pyran-4-one (A 1 )Synthesis
[0032] Ammonium acetate (15mg, 0.2mmol) was added to water (30mL) of guaiazulene (200mg, 1.0mmol) and 3-phenylkojic acid methanol (300mg, 1.2mmol), heated to reflux for 7 hours (using silica gel Chromatographic plate (TLC) to monitor the reaction). After the reaction was completed, the reaction mixture was cooled, filtered, washed with water, and dried to obtain a crude product. Ethanol recrystallization, yield 78%.
[0033] The structural analysis is as follows:
[0034]
[0035] 1 H NMR (400MHz, CDCl 3 )δ: 1.46(d, J=6.6Hz, 6H), 2.62(s, 3H), 3.05(s, 3H), 3.20~3.25(m, 1H), 4.20(s, 2H), 5.68(s, 1H ),6.36(s,1H),7.10-7.19(m,5H),7.28-7.30(m2H),7.78(s,1H),8.26(s,1H).
[0036] IR(KBr)ν:3412(OH),1641(C=O)cm -1 .
[0037] MS(ESI)m / z:429[M+H] + .
[0038] Elemental analysis (C 28 h 28 o 4 ): measured value (theoretical value), C 78.56 (78.48), H 6.67 (...
Embodiment 2
[0040] 2-(3,8-Dimethyl-6-isopropylazulene-1-yl)(4-methylphenyl)methyl-3-hydroxy-6-hydroxymethyl-4H-pyran-4-one (A 2 )Synthesis
[0041] Ammonium acetate (23mg, 0.3mmol) was added to guaiazulene (200mg, 1.0mmol) and 3-(4-methylphenyl)methylkojic acid methanol (312mg, 1.0mmol) in water (30mL) , heated to reflux for 7 hours (reaction monitored by silica gel chromatography (TLC)). After the reaction was completed, the reaction mixture was cooled, filtered, washed with water, and dried to obtain a crude product. Ethanol recrystallization, yield 80%.
[0042] The structural analysis is as follows:
[0043]
[0044] 1 H NMR (400MHz, CDCl 3)δ: 1.32(d, J=6.6Hz, 6H), 2.41(s, 3H), 2.60(s, 3H), 3.05(s, 3H), 3.21~3.27(m, 1H), 4.20(s, 2H ),5.68(s,1H),6.36(s,1H),7.19(d,J=8.0Hz,2H),7.23-7.27(m 2H),7.32(d,J=8.0Hz,2H),7.79( s,1H),8.34(s,1H).
[0045] IR(KBr)ν:3419(OH),1637(C=O)cm -1 .
[0046] MS(ESI)m / z:443[M+H] + .
[0047] Elemental analysis (C 29 h 30 o 4 ): measured valu...
Embodiment 3
[0049] 2-(3,8-Dimethyl-6-isopropylazulene-1-yl)(3-methoxyphenyl)methyl-3-hydroxy-6-hydroxymethyl-4H-pyran-4 Ketone (A 3 )Synthesis
[0050] Ammonium acetate (15mg, 0.2mmol) was added to guaiazulene (200mg, 1.0mmol) and 3-(3-methoxyphenyl)methylkojic acid methanol (360mg, 1.3mmol) in water (35mL) , heated to reflux for 8 hours (monitoring the reaction with silica gel chromatography (TLC)). After the reaction was completed, the reaction mixture was cooled, filtered, washed with water, and dried to obtain a crude product. Ethanol recrystallization, yield 81%.
[0051] The structural analysis is as follows:
[0052]
[0053] 1 H NMR (400MHz, CDCl 3 )δ: 1.31(d, J=6.6Hz, 6H), 2.64(s, 3H), 3.07(s, 3H), 3.21~3.25(m, 1H), 3.82(s, 3H), 4.23(s, 2H ),5.65(s,1H),6.33(s,1H),7.13(s,1H),7.18-7.20(m,3H),7.25-7.27(m 2H),7.85(s,1H),8.42(s ,1H).
[0054] IR(KBr)ν:3427(OH),1635(C=O)cm -1 .
[0055] MS(ESI)m / z:459[M+H] + .
[0056] Elemental analysis (C 29 h 30 o 5 ): measured va...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap