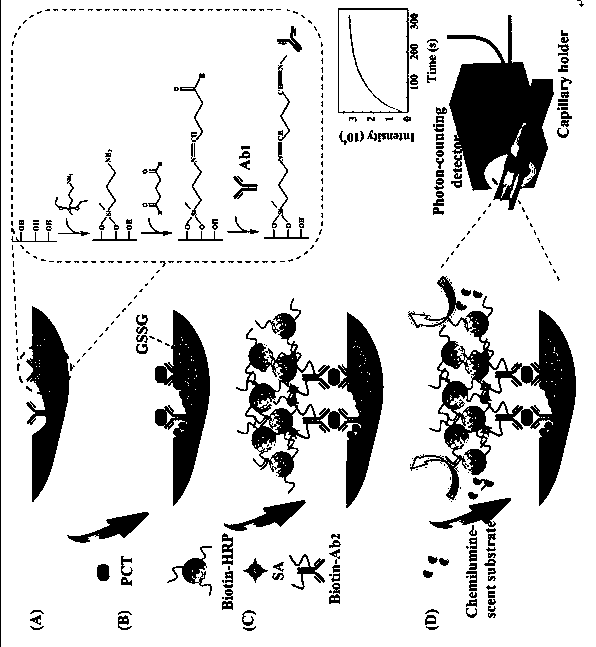
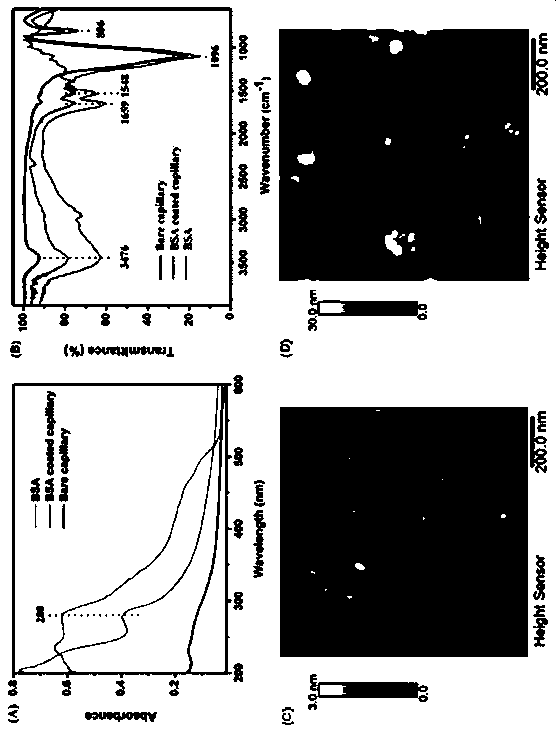
Novel signal amplification-capillary tube chemiluminescence immunosensor
A technique of chemiluminescence immunity and signal amplification, which is applied in the field of highly sensitive capillary chemiluminescence immunosensors, and can solve the problems of limited detection sensitivity of capillary immunosensors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 A Novel Signal Amplification-Capillary Chemiluminescence Immunosensor
[0032] 1. Pretreatment of capillary
[0033] Wash the capillary with 0.1M HCl and 0.1M NaOH in sequence for 20 min and 60 min respectively to activate the silicon hydroxyl groups on the inner surface; then wash with water, N 2 Blow dry; pass through 1% v / v aminopropyldiethoxymethylsilane (3-ADMS) toluene solution, make the inner surface of the capillary covered with a layer of amino groups through mutual reaction; wash with toluene, N 2 Blow dry, and then add 2.5% glutaraldehyde (GA) aqueous solution, the aldehyde group at one end interacts with the amino group on the inner wall of the capillary, and the aldehyde group at the other end is used to bind the coated antibody Ab 1 ;
[0034] 2. Antibody Ab 1 fixed
[0035] Passage 40 μg / mL procalcitonin Ab into the aldehyde-activated capillary 1 , sealed at both ends, placed in a refrigerator at 4°C overnight, washed with 0.1M pH 7.4 PBS s...
Embodiment 2
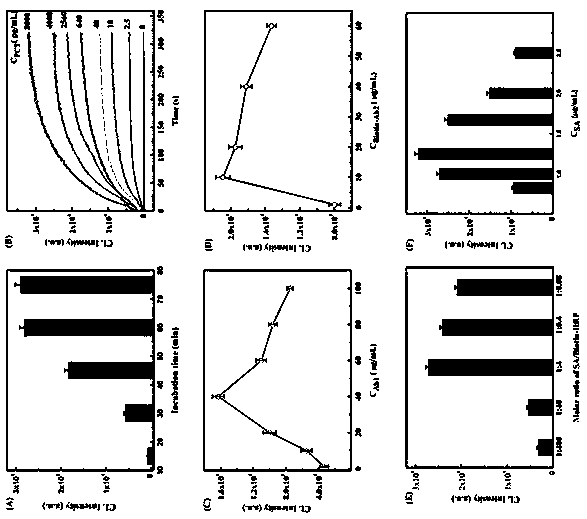
[0038] Example 2 Procalcitonin Antigen Standard Curve
[0039] 1. Optimization process and results
[0040] The standard curve was made under optimal conditions, and the condition optimization process was as follows: the incubation time of different immune reactions, Ab 1 Concentration, B-Ab 2 concentration, the ratio of streptavidin to biotin-labeled horseradish peroxidase, and the concentration of streptavidin on the signal intensity, and the conditions corresponding to the strongest signal were selected as the optimal conditions. The results are as follows image 3 shown.
[0041] 2. Standard curve
[0042] 1) Prepare a series of procalcitonin antigen standard substances with different concentrations into the sensor prepared in Example 1 with antigen diluent. Immunoreact at 37°C for 60 minutes. PBST (containing 0.05% v / v Tween-20 PBS) to wash away unbound PCT;
[0043] 2) Biotin-labeled procalcitonin antibody (B-Ab 2 ) into the sensor, immunoreact at 37°C for 60 minut...
Embodiment 3
[0048] Example 3 Interference Measurement
[0049] Use antigen diluent to prepare PCT solution with a concentration of 1000 ng / mL, and use it as a solvent to prepare a series of interfering reagents (ascorbic acid, glucose, leucine, glycine, glutamic acid, carcinoembryonic antigen, immune globulin G), the concentration ratio PCT: interfering reagent = 1:10; measure the chemiluminescence intensity according to the above method, and calculate the ratio of the chemiluminescent intensity with different interfering reagents to those without interfering reagents, as Figure 5 As shown, the inhibition or enhancement of the signal intensity detected by PCT ranged from -7.0% to +7.6%, indicating that the detection of procalcitonin was not affected in the presence of interfering reagents.
[0050] Embodiment 3 actual sample detection
[0051] 1. Sample detection method
[0052] 1) Pass the actual sample solution into the sensor prepared in Example 1, perform immunoreaction at 37°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com