A kind of phthalic dihydroxy polymer carrier and its application in the construction of drug complex nano-delivery system
A nano-drug delivery system and o-phthalic dihydroxy technology, applied in the field of pharmacy, can solve the problems of reducing the pharmacological activity of drugs and difficult prototype drugs, etc., achieve reversible release of drugs with drug loading, enhance drug stability, and improve therapeutic effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation and characterization of embodiment 1 polyethylene glycol-dopamine
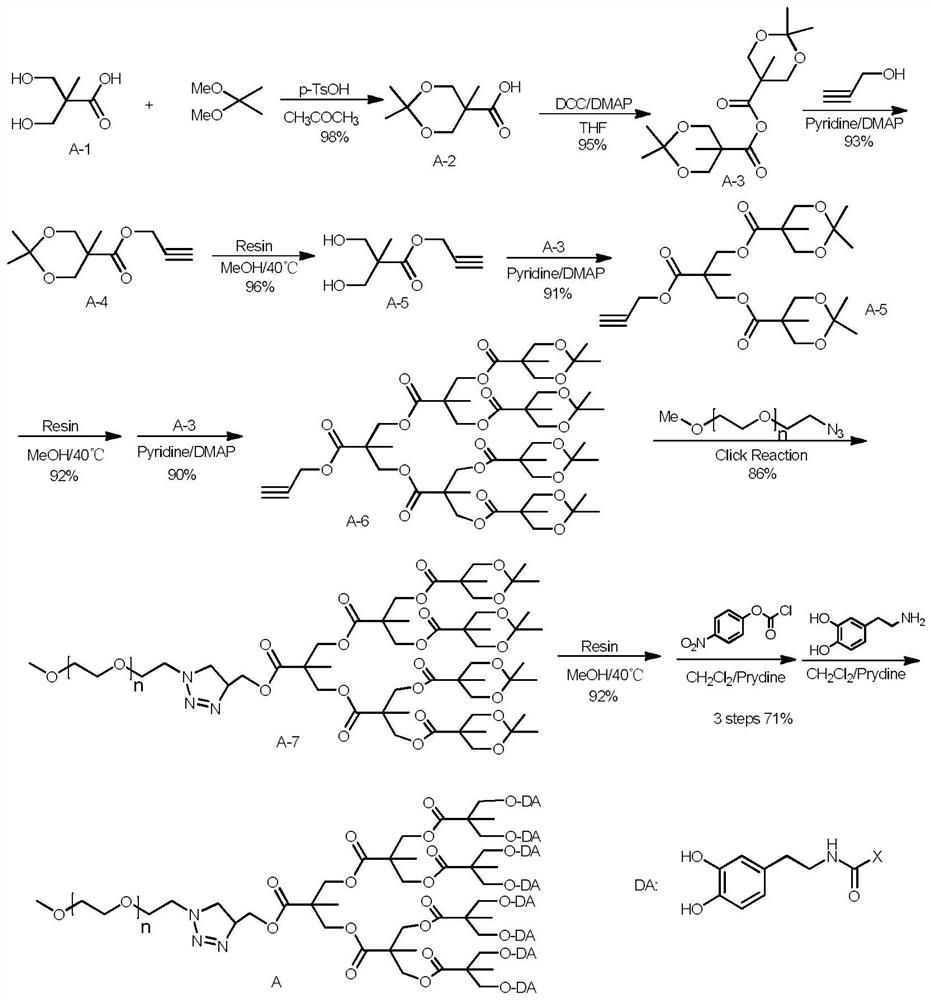
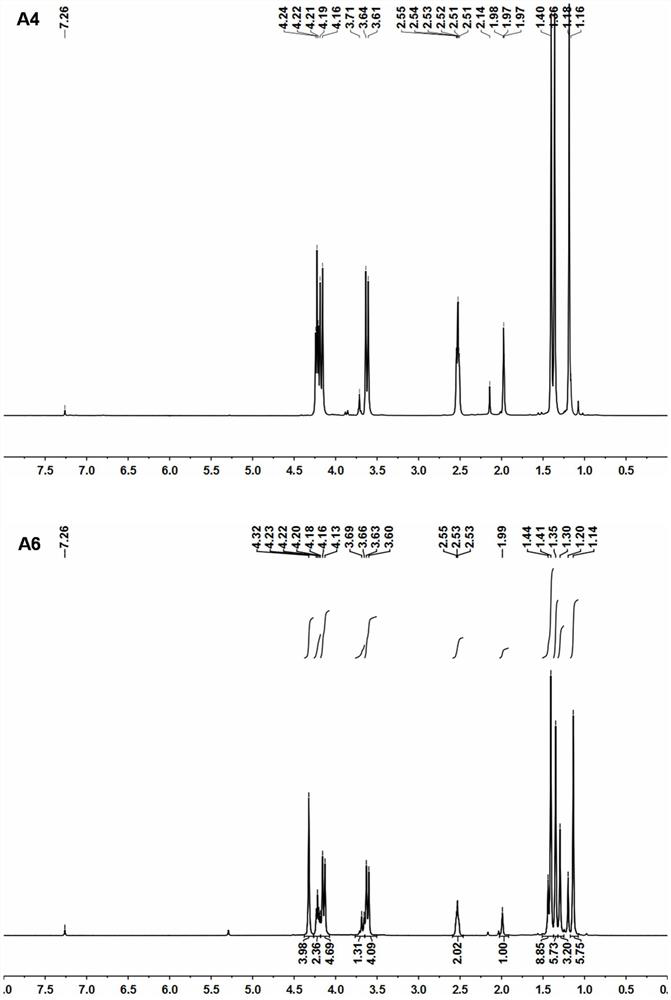
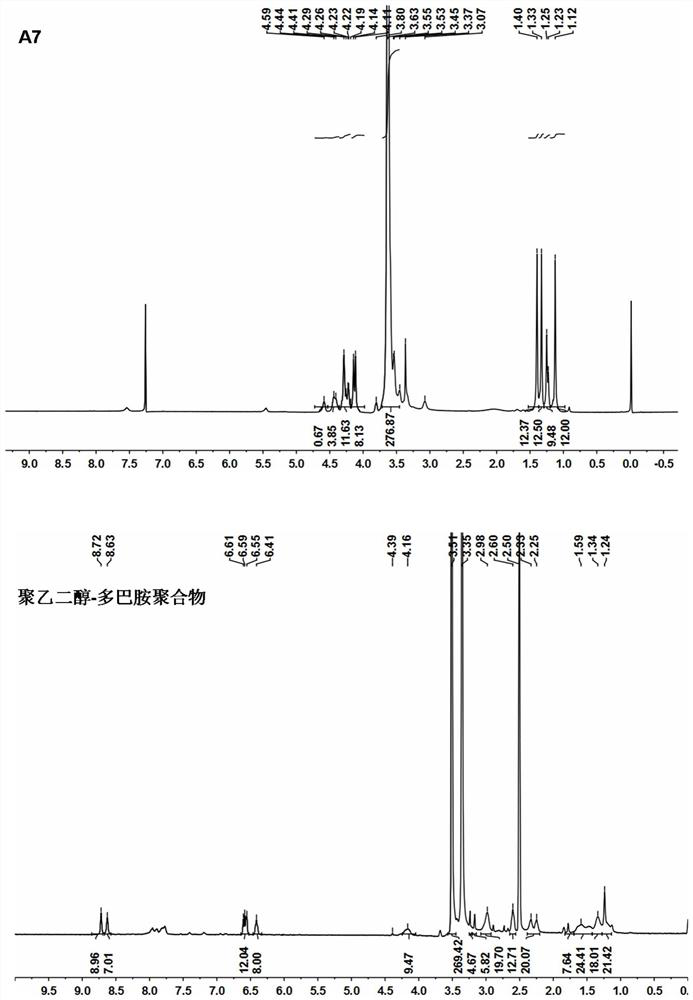
[0029] Synthetic roadmap as figure 1 As shown, the specific process is as follows:
[0030] Synthesis of Intermediate A-4:
[0031] Under nitrogen protection, weigh 1,1-dihydroxypropionic acid (50.0 g, 0.37 mol) and suspend it in 500 mL of acetone, add acetonide (46.5 g, 0.45 mol) and a catalytic amount of p-toluenesulfonic acid at room temperature Stir until the solution is clear, and spin to remove acetone to obtain a white solid. The resulting solid was dissolved in 500 mL of ethyl acetate, washed three times with saturated brine (3×50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 63.1 g of a white solid (98% yield). Dissolve 50 g of the above solid in 500 mL of tetrahydrofuran, add DCC (47.8 g, 0.23 mol) and DMAP (1.0 g, 0.01 mol) and stir at room temperature for 12 h, check by TLC until the raw material disappears, filter, concentrate the...
Embodiment 2
[0039] Embodiment 2 Preparation and characterization of bortezomib-polymer complex
[0040] Weigh the carrier material polyethylene glycol-dopamine (5.0mg, 8.62×10 -4 mmol) and bortezomib (2.65mg, 6.90×10 -3 mmol) was dissolved in 0.5mL chloroform, and after the solution was clarified, it was vortexed at room temperature for 1 h to form a bortezomib-polymer complex. 1 H-NMR characterizes its structure, showing that the structure is correct (attached image 3 ). 1 H-NMR characterization (solvent: DMSO-d6), the results are shown in the attached figure 2 , Visible: δ8.99 (2H, s) δ8.73-8.64 (4H, m), δ7.70-7.06 (13H, m), δ6.52 (2H, m), δ6.38 (2H, s) , δ6.11(5H, m), δ4.88(3H, m), δ4.69(3H, m), δ4.24(275H, s), δ2.89(9H, m) δ1.54-1.16 (21H, s), δ1.02-0.98 (5H, s), δ0.76-0.71 (9H, m).
Embodiment 3
[0041] Example 3 Preparation and Characterization of Nanoparticles Constructed by Bortezomib-Polymer Complex
[0042] Polyethylene glycol-dopamine (5.0mg, 8.62×10 -4 mmol) and bortezomib (2.65mg, 6.90×10 -3 mmol) was dissolved in 0.5 mL chloroform, and after the solution was clear, vortexed at room temperature for 1 h. Add 1 mL of 0.5% sodium cholate solution to the system, emulsify and ultrasonically form an O / W emulsion, and slowly add it dropwise to 5 mL of 10 mmol / L phosphate buffer saline (PBS, pH 7.6) at a stirring rate of 600 rpm. After dropping, continue to stir for 4h, concentrate under reduced pressure to remove chloroform, centrifuge 3 times (rotating speed 2500rpm / min), discard the supernatant, PBS is dispersed, and the volume is set to 1mL to prepare nanoparticles, and the obtained nanoparticles are rounded. Uniform size (such as Figure 4 Shown in A), the drug loading capacity of its bortezomib is 7.9%;
[0043] The in vitro release experiments of nanoparticl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com