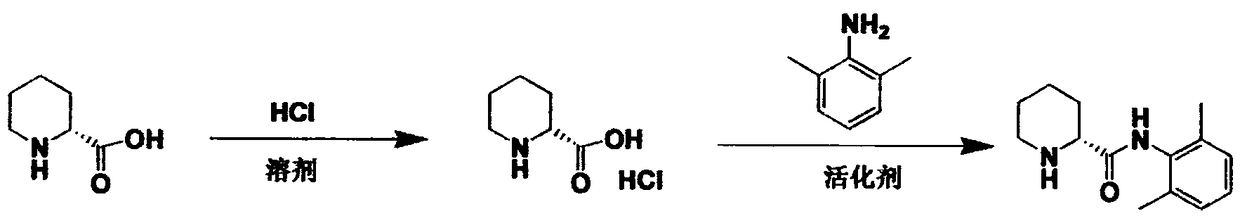
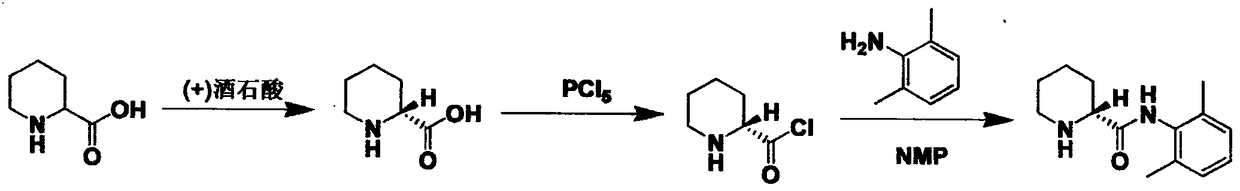
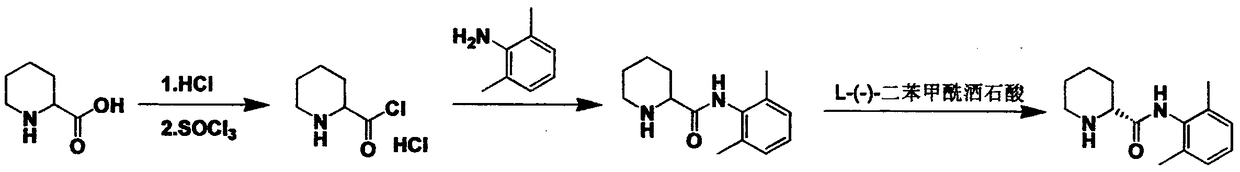
Method for preparing ropivacaine intermediate
A technology for ropivacaine and intermediates, which is applied in the field of preparation of intermediates, can solve problems such as being dangerous and unsuitable for industrial production, and achieve the effects of simple operation, increased yield, and simplified process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Mix 2.65g of L-piperidine-2-carboxylic acid with 13ml of toluene, slowly inject HCl gas under stirring at room temperature until the pH of the reaction solution is 3, then add 2.42g of 26-dimethylaniline, and then dropwise add 9.2g of trichloro Oxyphosphorus, the dropwise addition is completed, and the temperature is raised to 50°C for 6 hours. After the reaction is completed, the temperature is lowered to 10°C, and then 13ml of purified water is added dropwise, and then the pH is adjusted to 12 with 40% sodium hydroxide solution, and then extracted with 13ml of ethyl acetate for 3 The second time, the organic phase was collected, concentrated and spin-dried, and dried at 40° C. to obtain a white solid 3.6 with a yield of 77%. The HPLC control was the same point.
Embodiment 2
[0025] Mix 2.65g of L-piperidine-2-carboxylic acid with 13ml of DMP, slowly inject HCl gas under stirring at room temperature until the pH of the reaction solution is 3, then add 2.42g of 26-dimethylaniline, and then dropwise add 9.2g of oxytrichloride Phosphorus, the dropwise addition is completed, the temperature rises to 50°C, TLC controls the formation of product spots, reacts for 3 hours, after the reaction is completed, cools down to 10°C, then adds 26ml of purified water dropwise, and then adjusts the pH to 5-6 with 40% sodium hydroxide solution. Then extract 3 times with 13ml of ethyl acetate, collect the aqueous phase, adjust the pH=12 with 40% sodium hydroxide solution, then crystallize at 0-5°C for 3h, collect the solid, and separate by chromatography column, DCM:MeOH=10: 1. Obtained 0.72 g of white solid with a yield of 15.4%. The HPLC control was the same point.
Embodiment 3
[0027] Mix 2.65g of L-piperidine-2-carboxylic acid with 13ml of dioxane, slowly inject HCl gas under stirring at room temperature until the pH of the reaction solution is 3, then add 2.42g of 26-dimethylaniline, and then dropwise add 9.2g Phosphorus oxychloride, after the dropwise addition was completed, the temperature was raised to 50°C, and the product point was generated as compared with TLC, and reacted for 12 hours. After the reaction was completed, the temperature was lowered to 10°C, and then 13ml of purified water was added dropwise, and then the pH was adjusted to 12 with 40% sodium hydroxide solution. , and then extracted 3 times with 13ml of ethyl acetate, collected the organic phase, concentrated and spin-dried, and dried at 40°C to obtain a white solid 3.6, with a yield of 77%. The HPLC control was the same point.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com