Synthesis and application of quinoline-2(1H) ketopiperazines compound
A technology of ketone compounds and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
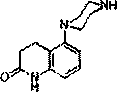
[0031] 5-(pyrazin-1-yl)-3,4-dihydroquinoline-2(1 H ) ketone (2)
[0032]
[0033] Add 5-amino-3,4-dihydroquinoline-2 (1 H ) ketone 7 (5.00 g, 30.83 mmol), bis-(2-bromoethyl) amine hydrobromide (50.57 g, 33.91 mmol), stirred and warmed up to 80 ° C, and added dropwise three batches of hydroxide within half an hour Potassium (1.90 g, 33.91 mmol, 10 ml) in water. After the addition, the reaction was warming up to 100 ° C, and then incubated for 3 hours. After the reaction was completed, it was cooled to room temperature, filtered, and the filtrate was adjusted to a pH of 10 with 10% potassium hydroxide aqueous solution, and a large amount of white solids were separated out. Suction filtration, washing with an appropriate amount of water, and drying gave white powdery solid 8 (6.4 g, yield 61%, Mp 235-237 °C); 1 H NMR (300 MHz, DMSO-d 6 ) δ: 10.01 (s, 1H, quinolin, -CONH-), 7.07(t, J = 7.9 Hz, 1H, quinolin-H 7 ), 6.65 (d, J = 8.0 Hz, 1H, quinolin-H 8 ), 6.58(d, J =...
Embodiment 2
[0035] 5-(4-(oxirane-2-yl-methyl)pyrazin-1-yl)-3,4-dihydroquinoline-2(1 H ) ketone (3)
[0036]
[0037] Add 5-(pyrazin-1-yl)-3,4-dihydroquinoline-2(1 H) ketone 8 (6.2 g, 29.07 mmol), potassium iodide (0.12 g, 0.73 mmol), tetra-tert-butylammonium bromide (0.24 g, 0.73 mmol), stirred at room temperature until completely dissolved, then added epichlorohydrin (5.38 g , 58.14 mmol), after stirring for about 10 minutes, a white colloidal solid was precipitated. Stir the reaction at room temperature for 4 hours. After the reaction is complete, a creamy white solid sinks in the bottom of the bottle. Remove the upper aqueous layer, add ethyl acetate to crystallize, filter, and dry to obtain white powdery solid 9 (5.3 g, yield 64%, Mp 184-186 °C); 1 H NMR (300 MHz, DMSO-d 6 ) δ: 10.02 (s, 1H, quinolin,-CONH-), 7.08 (t, J = 7.9 Hz, 1H, quinolin-H 7 ), 6.69 (d, J = 7.9 Hz, 1H, quinolin-H 8 ), 6.60 (d, J = 7.8 Hz, 1H, quinolin-H 6 ), 3.05 (m, 1H, CH), 2.76-2.87 (m, 6H, -CH...
Embodiment 3
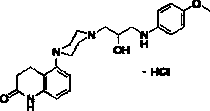
[0039] 5-(4-(2-Hydroxy-3-((4-methoxyphenyl)amino)propyl)piperazin-1-yl)-2-oxo-1,2,3,4-tetrahydroquinone Phenyl hydrochloride (1a)
[0040]
[0041] 5-(4-(oxirane-2-yl-methyl)pyrazin-1-yl)-3,4-dihydroquinoline-2(1 H ) ketone 9 (0.20 g, 0.7 mmol) and p-methoxyaniline (0.17 g, 1.4 mmol) were mixed uniformly and then heated to 90 °C until the reaction was molten, and stirred for 0.5 hours. Cooled and passed through the column to obtain a colorless oily liquid. After the oil was acidified with concentrated hydrochloric acid, it was recrystallized from a methanol-acetone mixed solvent to obtain 10a as a white powdery solid (yield 75%, Mp 258-261 °C); 1 H NMR (300 MHz, CDCl 3 ) δ: 7.99 (s, 1H, quinolin, -CONH-), 7.17 (t, J = 7.9 Hz, 1H, quinolin-H 7 ), 6.80(m, 3H, quinolin-H 8 and Ar-H 2 , H 6 ), 6.65 (d, J = 8.8 Hz, 2H, Ar-H 3 , H 5 ), 6.55(d, J = 7.8 Hz, 1H, quinolin-H 6 ), 4.05 (m, 1H, NH), 3.77 (s, 3H, CH 3 O-), 3.17-3.34 (m, 1H, CH), 2.78-3.14 (m, 10H, -CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com