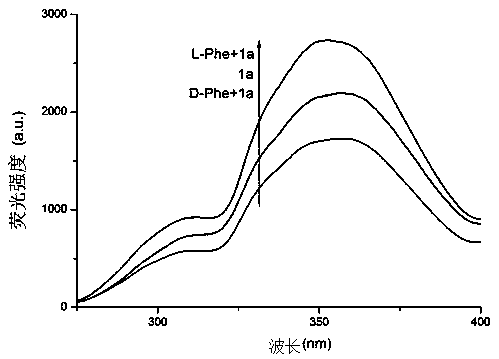
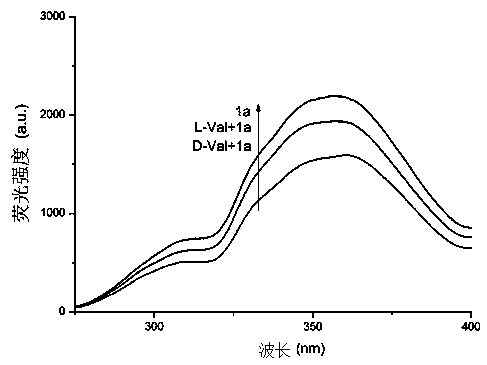
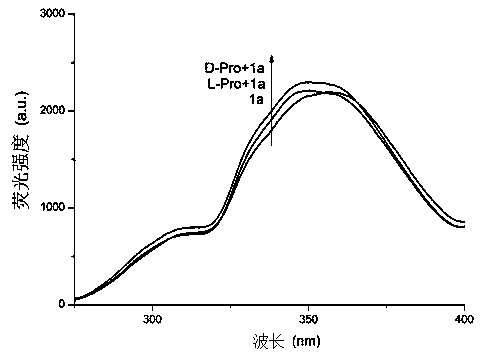
Chiral probe compound based on square amide skeleton and synthesis of compound and application of fluorescence recognition of amino acid enantiomers
A fluorescence recognition and compound technology, which is applied in the field of chemical synthesis and chiral recognition detection, can solve the problems of long synthetic route, complex structure and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Synthesis of probe compound a
[0043] (1) Synthesis of intermediate compounds: Add dimethyl squarylate (10.0 mmol) and 10 mL of dry anhydrous methanol into a 50 mL reactor, and stir until the dimethyl squarylate is completely dissolved in methanol. Dissolve p-methylaniline (10.0mmol) in 5ml of methanol and add it dropwise slowly. During the dropwise addition, a yellow substance precipitates. After adding p-methylaniline, stir at room temperature for 10 minutes. Filter and wash with anhydrous methanol several times to obtain a filter residue as a pale yellow solid. After the mother liquor was concentrated by half, it was frozen for 2 h and then filtered again, washed with anhydrous methanol several times to obtain the filter residue. 1.7g of the product was obtained for the first time, and 0.21g of the product was obtained for the second time. A total of 1.91g of the product was obtained, with a yield of 88%. Its synthetic formula is:
[0044]
[0045] (2) Synt...
Embodiment 2
[0056] 1. Synthesis of probe compound b
[0057] (1) Synthesis of intermediate compounds: same as Example 1;
[0058] (2) Synthesis of tert-butyl phenylalanine: Dissolve L-phenylalanine (20.0mmol) in tert-butyl acetate in 100mL, cool in an ice bath, slowly add perchloric acid (30mmol) dropwise under stirring, drop After completion, it was naturally raised to room temperature and stirred for 12 h. The reaction solution was washed with water and 1.0mol / L hydrochloric acid successively, then the pH value was adjusted to 9 with 10% potassium carbonate aqueous solution, extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated, and the obtained crude product was used for The yellow oily substance tert-butyl phenylalanine was separated by column chromatography, and the yield was 69%. Its synthetic formula is as follows:
[0059]
[0060] (3) Synthesis of probe compound b: Add the intermediate compound (1.5mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com