Preparation method and application of antitumor protein
A protein and anti-tumor drug technology, applied in the field of anti-tumor protein preparation, can solve the problems of huge difficulty in extracting protein and difficult to obtain efficiently
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The construction of embodiment 1PvEXP100 recombinant plasmid
[0023] Prokaryotic expression plasmid Pet28a(+), host strain BL21(DE3) and IPTG for induction were purchased from Beijing Quanshijin Biotechnology Co., Ltd.; restriction enzymes, T4 DNA ligase, pfu DNA polymerase and dNTP were purchased from TakaRa Corporation. The synthesis of primers and nucleotide sequence sequencing were completed by Suzhou Jinweizhi Biotechnology Co., Ltd. Agarose affinity medium nickel column (Ni) was purchased from QIAGEN; His-Taq tag antibody was purchased from Cell Signaling Technology.
[0024] Design primers to obtain the gene sequence of the Plasmodium vivax PvEXP100 protein by PCR. The primers are as follows: SEQ ID NO.3: GGATCCATGTTCTGGAAAGTAAAGGGG; SEQ ID NO.4: CTCGAGCAAAAGAAGGGCAACCATCAG, where GGATCC is the restriction site BamHI of SEQ ID NO.3; CTCGAG is Restriction site XhoI of SEQ ID NO.4.
[0025] Using the Plasmodium ovale genome as a template, the PvEXP100 gene sequ...
Embodiment 2
[0028] Embodiment 2 Expression of recombinant protein PvEXP100
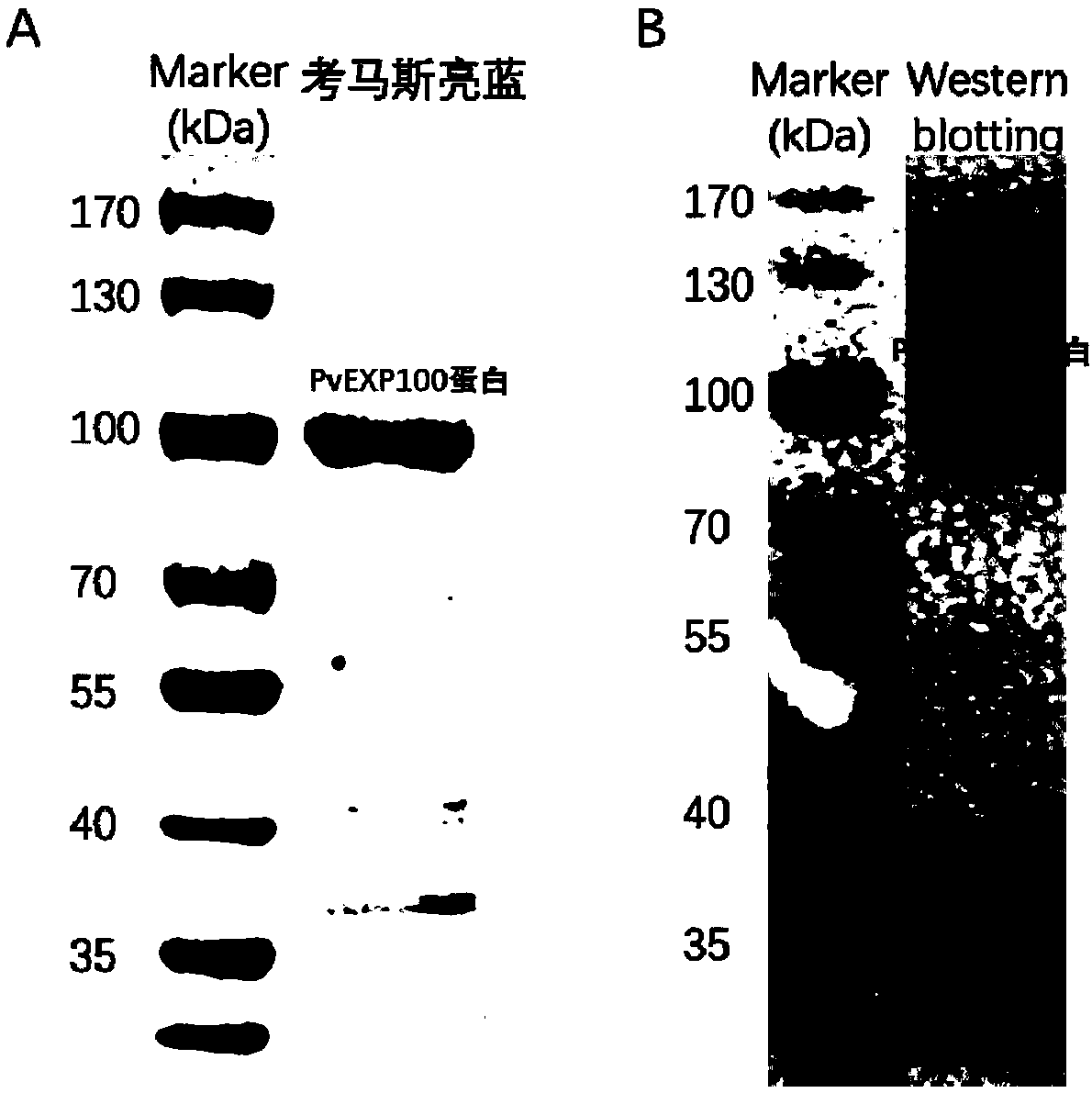
[0029] Inoculate 5ml of LB medium containing kanamycin with the positive single clone with correct sequencing, culture overnight at 37°C, inoculate the bacterial liquid into 500ml fresh LB medium containing kanamycin, when OD 600 When it is 0.6-0.8, add 1mmol / L IPTG, induce 8h. The induced PvEXP100 was sonicated and lysed, and analyzed by 10% SDS-PAGE electrophoresis, which showed that the PvEXP100 protein was mainly located in the inclusion body, and the molecular weight was consistent with the expectation.
[0030] Use 8M urea to dissolve inclusion bodies to release PvEXP100 protein, which has a His-tag tag at the carbon terminus. Therefore, use GE’s His-tag nickel column and carry out Ni 2+ Affinity chromatography purification. The protein was purified with different concentrations of imidazole. The concentrations of imidazole were 20mM, 50mM, 100mM, 150mM and 250mM. The protein washed with 150mM imidazole w...
Embodiment 3
[0032] Example 3 PvEXP100 protein anti-tumor activity identification
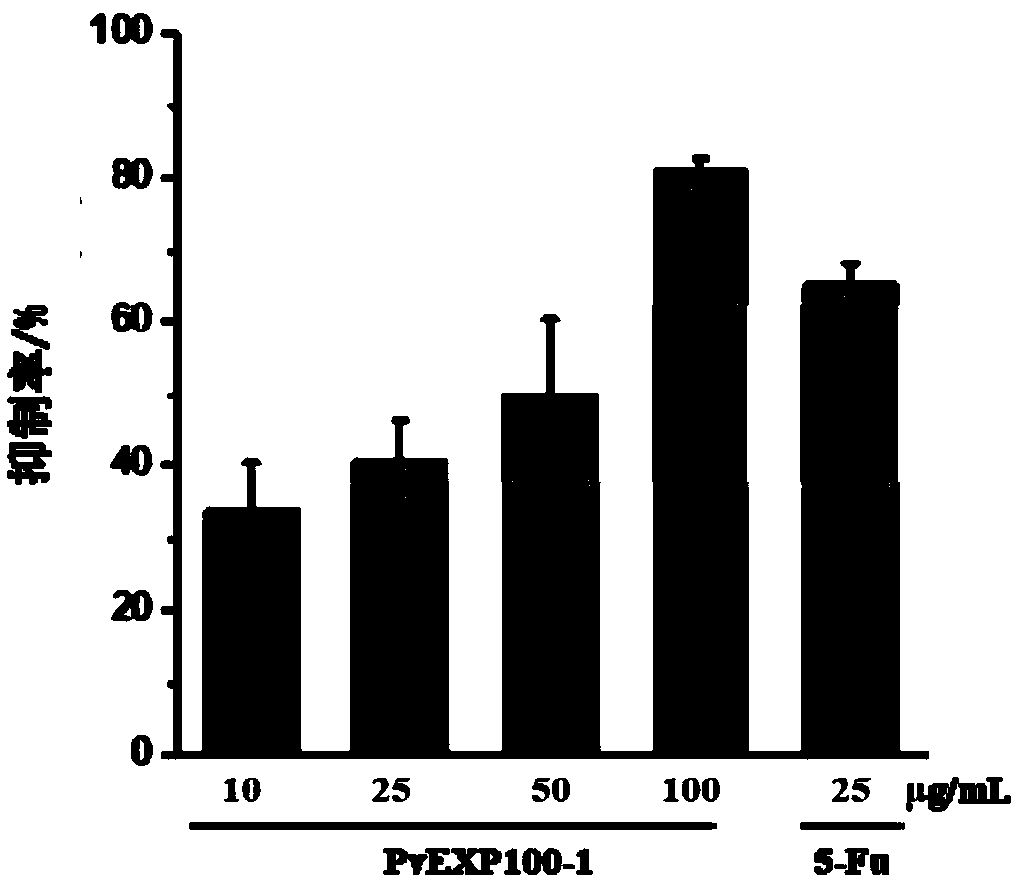
[0033] (1) CCK8 method to detect the inhibitory effect of PvEXP100 on tumor cell proliferation
[0034] Take HepG2 in the logarithmic phase and use DMEM medium containing 10% FBS to adjust the cell concentration to 5×10 4 / mL, inoculated in 96-well plate with 100 μL per well, placed in 5% CO 2 , cultured in a 37°C incubator for 6 hours, discarded the supernatant after the cells adhered to the wall, washed with PBS buffer to remove non-adhered cells. 100 μL of drugs with different concentrations (10, 50, 100 μg / mL) were added to the sample group, and an equal volume of medium was added to the blank group. After continuing to culture for 24 hours, add 10 μL CCK8 solution to each well, continue to culture in the incubator for 2 hours, and measure the absorbance at 450 nm. A dose of 25 μg / mL antineoplastic drug 5-Fu was used as the control. Cell proliferation rate (%)=experimental group A450 / control group A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com