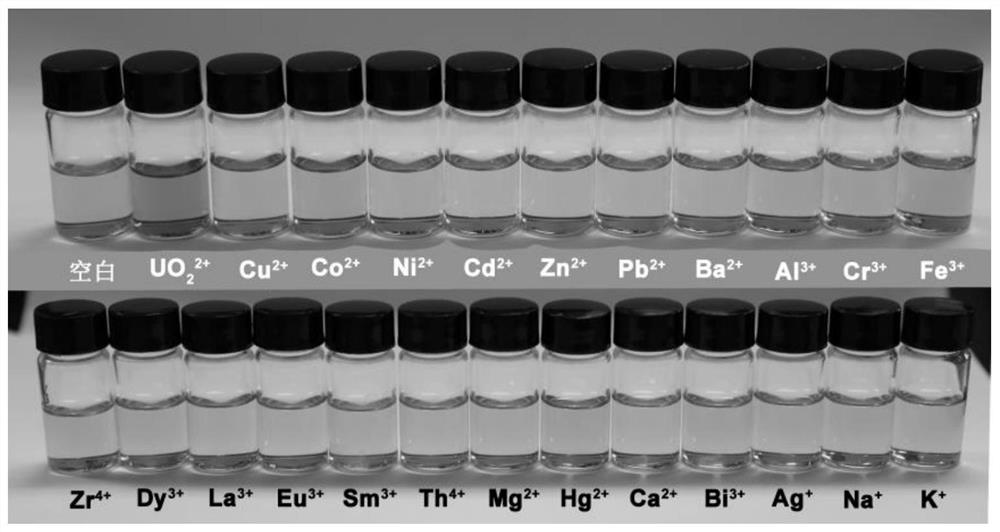
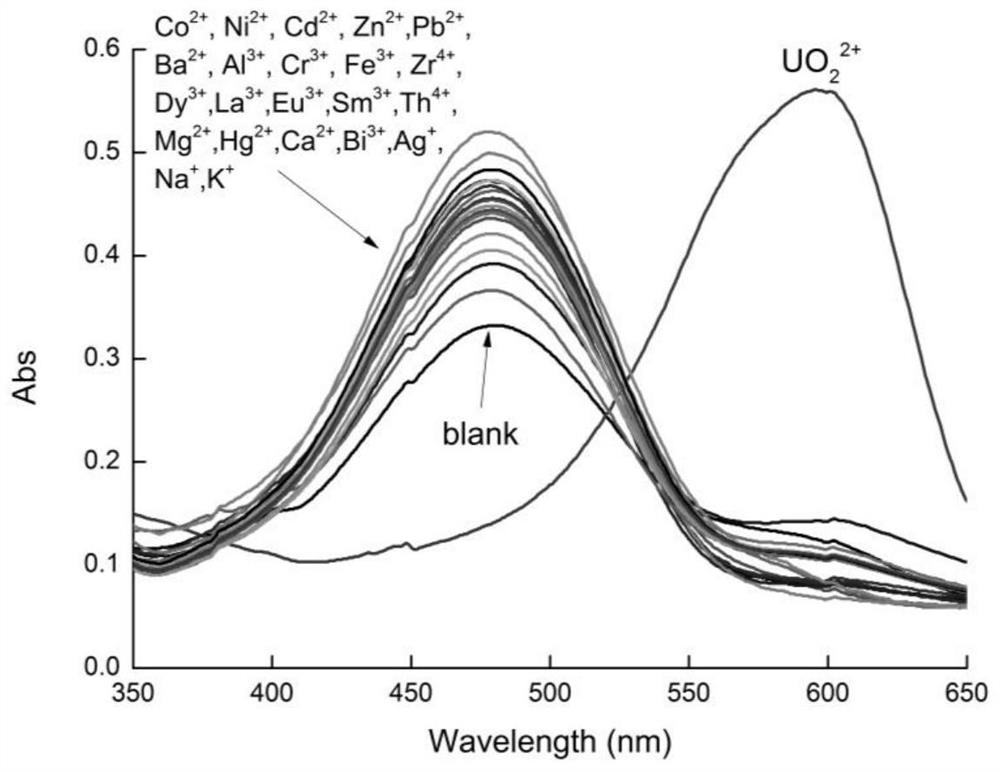
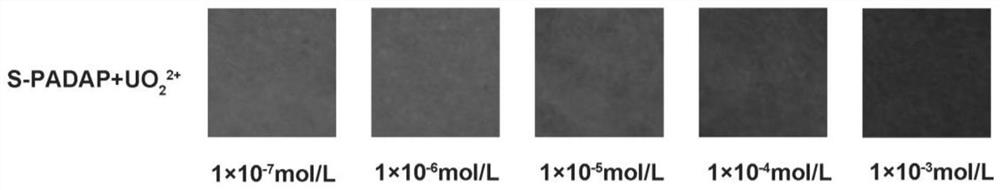
2-(5-bromo-2-pyridylazo)-5-diethylaminophenol derivative, its preparation method and application
A technology of diethylaminophenol and pyridine azo, applied in the field of analysis, can solve the problems of tedious sample preparation, inability to realize real-time online detection and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention also provides a method for preparing the above-mentioned 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol derivative, comprising the following steps:
[0053] A) mixing triphenylamine 4-boric acid, potassium carbonate, tetrakis(triphenylphosphine)palladium and solvent to obtain the first mixed solution;
[0054] B) reacting a solution of 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol with the first mixed solution at 80-110°C to obtain a second mixed solution;
[0055] C) quenching the second mixed solution with water to obtain a 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol derivative having a structure shown in formula (I);
[0056] Said step A) and step B) are all carried out under the condition of protective gas.
[0057] In the present invention, 4-boric acid triphenylamine and 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol are main reaction raw materials, and described 4-boric acid triphenylamine and 2-(5- The mass ratio of bromo-2-pyridylazo)-5-...
Embodiment 1
[0114] Under nitrogen atmosphere, 4-triphenylamine borate, potassium carbonate solid, tetrakis (triphenylphosphine) palladium and solvent are mixed, obtain the first mixed solution, wherein, potassium carbonate solid and tetrakis (triphenylphosphine) palladium The mass ratio of 1,4-triphenylamine borate to tetrakis(triphenylphosphine)palladium is 1:1.5, and the solvent includes 1,4-dioxane and water with a volume ratio of 2:1 , the dosage ratio of the 4-triphenylamine borate to the solvent is 30mg:2mL.
[0115] Continue to raise the temperature of the first mixed solution to 90° C. under nitrogen atmosphere, and then add the solution of 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol dropwise into the warmed second mixed solution. A mixed solution was reacted at 90° C. for 6 h to obtain a second mixed solution. The mass ratio of the 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol to 4-triphenylamine borate is 1:0.8, and the 2-(5-bromo-2-pyridylazo The solvent in the solution of ...
Embodiment 2
[0119] Under nitrogen atmosphere, 4-triphenylamine borate, potassium carbonate solid, tetrakis (triphenylphosphine) palladium and solvent are mixed, obtain the first mixed solution, wherein, potassium carbonate solid and tetrakis (triphenylphosphine) palladium The mass ratio is 4:1.5, the mass ratio of 4-triphenylamine borate to tetrakis(triphenylphosphine)palladium is 1:1, and the solvent includes N,N-dimethylformamide and N,N-dimethylformamide with a volume ratio of 3:2 Water, the ratio of the amount of triphenylamine 4-borate to the solvent is 30mg: 1mL.
[0120] Continue to raise the temperature of the first mixed solution to 110° C. under nitrogen atmosphere, and then add the solution of 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol dropwise to the heated second mixed solution. The first mixed solution was reacted at 110° C. for 6 h to obtain the second mixed solution. The mass ratio of the 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol to 4-boric acid triphenylamine is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com