Phenolic hydroxy epoxidation preparation technology
A phenolic hydroxyl ring and preparation process technology, applied in the field of phenolic hydroxyl epoxidation preparation process, can solve the problems of large environmental pollution, reduced hydrolyzable chlorine content, low epoxy products and the like, achieve process stability and reduce hydrolyzable chlorine content and inorganic chlorine content, the effect of reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
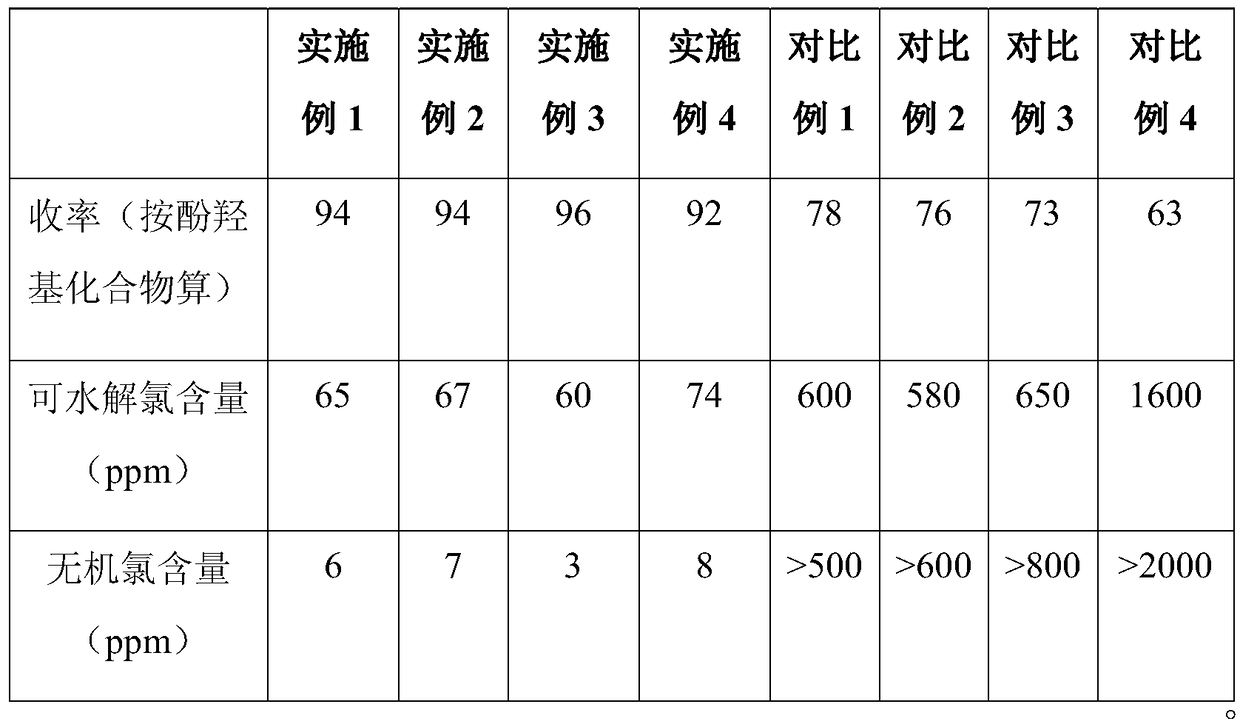
Examples
Embodiment 1
[0048] Soak 5g of SBA-15 mesoporous molecular sieve in 100mL aminopropyltriethoxysilane coupling agent hydrolyzate (concentration is 1wt%, adjust pH to 5 with glacial acetic acid and let stand for 2 hours); take out after soaking for 2 hours Dry;
[0049]Mix benzyltriethylammonium chloride aqueous solution (concentration: 20g / L, 100mL) with treated SBA-15 mesoporous molecular sieve (5g), remove the water by evaporation, and place the remaining material in an ultrasonic microwave device for 120 ℃ and heated for 20 minutes to obtain the SBA-15 mesoporous molecular sieve catalyst solidly loaded with benzyltriethylammonium chloride.
[0050] Add bisphenol A and epichlorohydrin (molar ratio 1:2.1) under normal pressure, add the mesoporous molecular sieve catalyst (addition molar amount is bisphenol 0.005 of A), carry out etherification ring-opening reaction at 100°C, the reaction time is 2 hours, and obtain chlorohydrin ether; when the system cools down to about 60°C, the catalyst...
Embodiment 2
[0052] The raw materials and process parameters of the phenolic hydroxyl epoxidation preparation process are exactly the same as in Example 1, the only difference being that the catalyst used is the SBA solid-loaded with benzyltriethylammonium chloride that has been used 5 times and recovered in Example 1. -15 mesoporous molecular sieve catalyst. The performance test results of the final product are listed in Table 1 below.
Embodiment 3
[0054] Soak 10g of SBA-15 mesoporous molecular sieve in 500mL aminopropyltrimethoxysilane coupling agent hydrolyzate (3% concentration, adjust pH to 5 with glacial acetic acid and let it stand for 1 hour); take it out and dry it after soaking for 2 hours;
[0055] Mix benzyltriethylammonium chloride aqueous solution (concentration: 1000g / L, 50mL) with treated SBA-15 mesoporous molecular sieve (10g), remove the water by evaporation, and place the remaining material in an ultrasonic microwave device for 140 ℃ and heated for 40 minutes to obtain the SBA-15 mesoporous molecular sieve catalyst solidly loaded with benzyltriethylammonium chloride.
[0056] Add bisphenol F and epichlorohydrin (molar ratio 1:2) under normal pressure, add the mesoporous molecular sieve catalyst (addition molar amount is bisphenol 0.005 of A), etherification ring-opening reaction was carried out at 110°C, and the reaction time was 1 hour to obtain chlorohydrin ether; when the system was cooled to about 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com