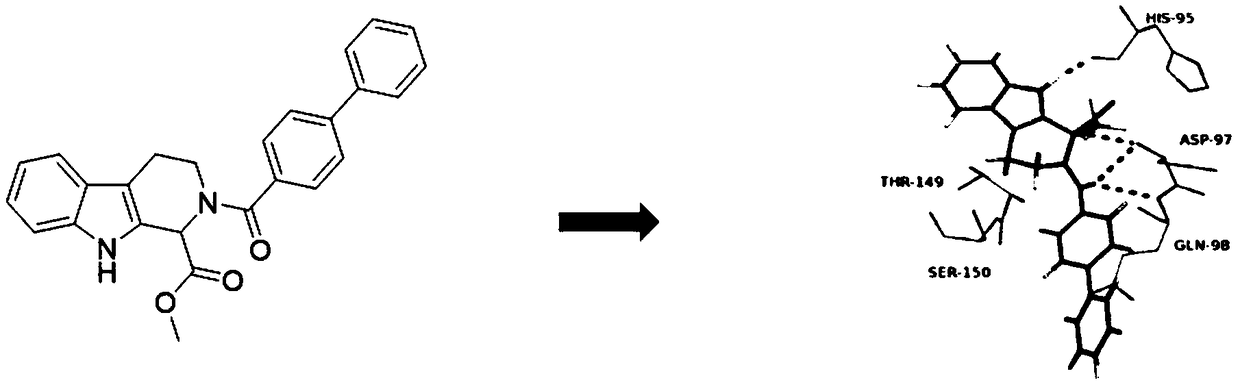
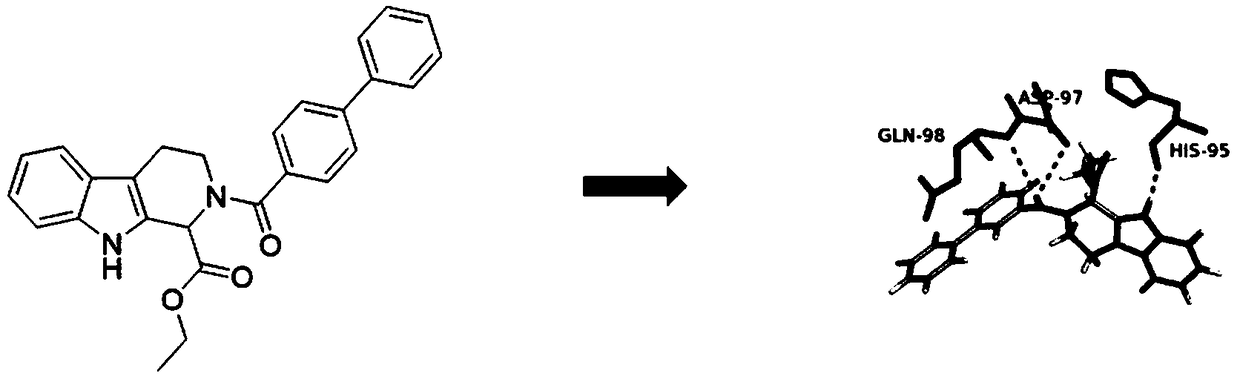
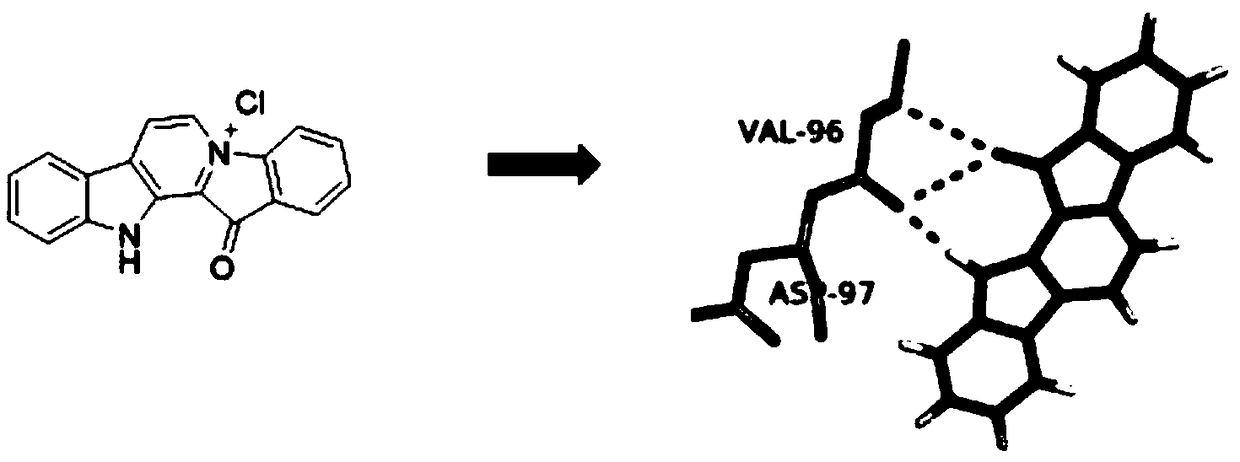
Preparation method and application of CDK4
A compound and general formula technology, applied in the field of preparation of tetrahydro-β-carboline compounds, can solve problems such as poor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The present invention will be explained in detail below in conjunction with the examples of compound preparation and pharmacological experiments.
[0024] 1. Compound Preparation
[0025] 1) Preparation of compound of general formula (I) Preparation of compound
[0026] a: Dissolve 6.00g of tryptamine in 100mL of water, add concentrated hydrochloric acid drop by drop until the solid is completely dissolved, add 4 equivalents of glyoxylic acid and 10% KOH solution dropwise at room temperature and constant pressure to adjust the pH to about 3-4. React at room temperature for 4 hours until the solid precipitates completely, monitor by thin layer chromatography until the reaction is complete, filter with suction, wash the filter cake with water three times, and recrystallize with methanol to obtain the intermediate 1,2,3,4-tetrahydro-β-carboline-1-carboxylate Acid 7.05g.
[0027] b: Dissolve 2.4mmol (0.5g) of benzoic acid with different substituents in 5ml of dry THF, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com