A sacubitril valsartan sodium pharmaceutical composition and preparation method thereof
A technology of sacubitril and sartan sodium, which is applied in directions such as drug combinations, pharmaceutical formulations, and active ingredients of heterocyclic compounds, can solve the problems of overall performance control of unfavorable products, poor reproducibility, unbalanced effects, etc., and achieves beneficial effects. The effect of large-scale industrial application, reduction of differences between wafers, and reduction of process costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
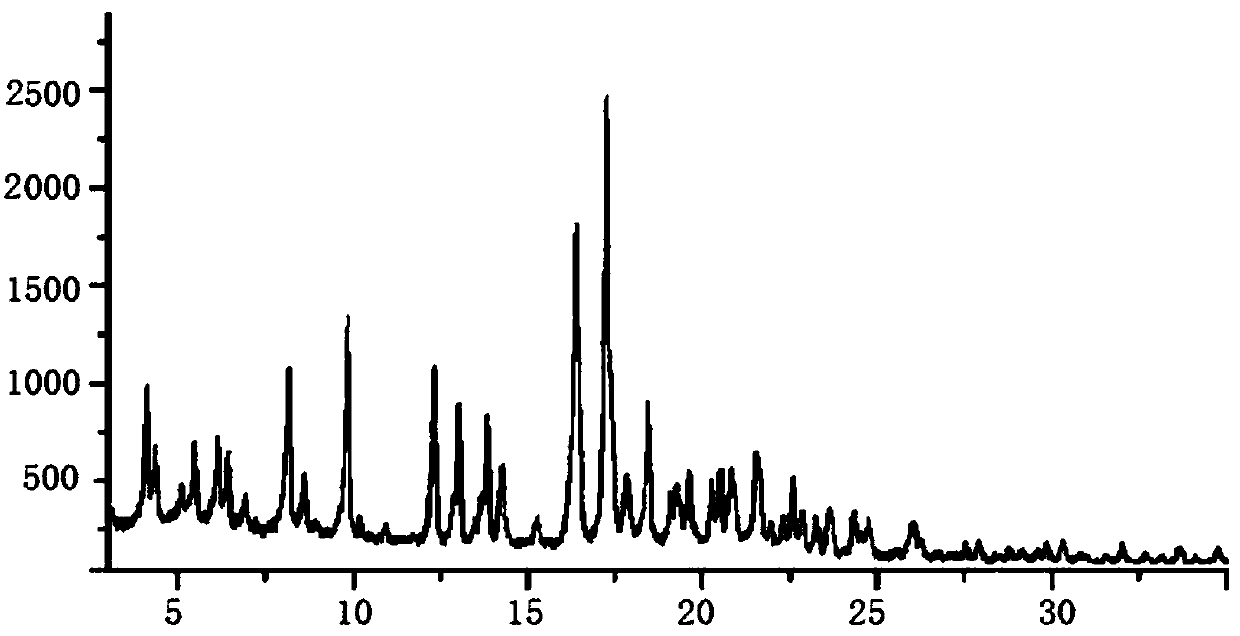
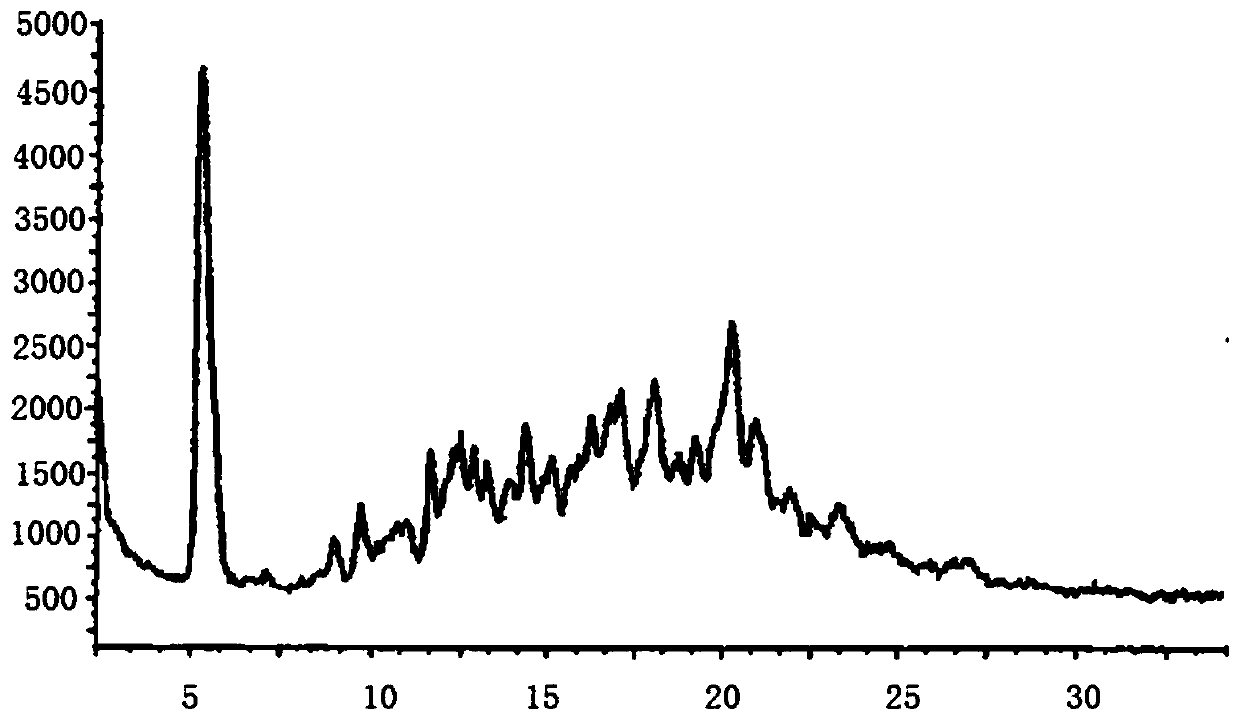
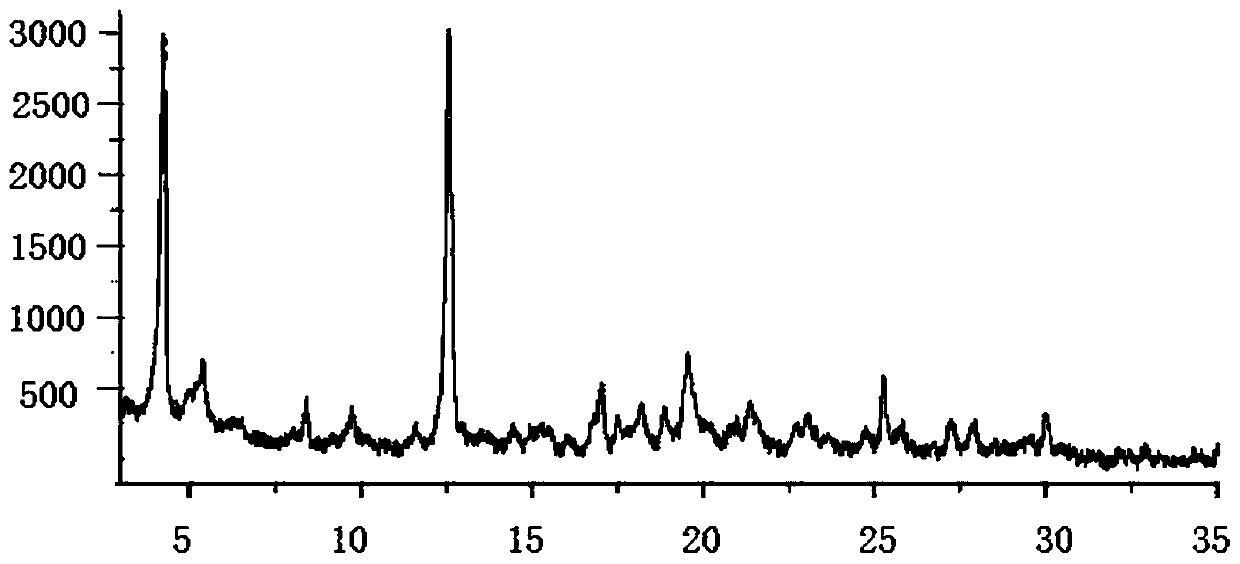
Image
Examples
Embodiment 1
[0057] A kind of sacubitril valsartan sodium pharmaceutical composition and preparation method thereof, comprising:
[0058]
[0059] (1) Add sacubitril-valsartan sodium and a small amount of acetone to make wet particles over 20 mesh, LCZ696:acetone=1:0.15g / mL;
[0060] (2) Pass the hydrophilic filler-sorbitol, the binder-hypromellose, and the disintegrant-croscarmellose sodium through a 80-mesh sieve, and add a small amount of acetone to make 20-mesh wet particles , the sum of the mass of hydrophilic filler, binder and disintegrant: acetone=1:0.5g / mL;
[0061] (3) Mix the wet granules of steps (1) and (2) evenly, granulate, then dry the granulated granules under reduced pressure at 30-40°C for 30 minutes, remove acetone, then add lubricant and mix well, Tablets were compressed according to the required specifications of the drug, coated with Opadry, and the weight was increased by 1%.
Embodiment 2
[0063] A kind of sacubitril valsartan sodium pharmaceutical composition and preparation method thereof, comprising:
[0064]
[0065] (1) Add sacubitril-valsartan sodium and a small amount of acetone to make wet particles over 20 mesh, LCZ696:acetone=1:0.15g / mL;
[0066] (2) Pass the hydrophilic filler-mannitol, the binder-hypromellose, and the disintegrant-cross-linked carboxymethyl starch sodium through a 80-mesh sieve, and add a small amount of acetone to make 20-mesh wet particles , the sum of the mass of hydrophilic filler, binder and disintegrant: acetone=1:0.5g / mL;
[0067] (3) Mix the wet granules of steps (1) and (2) evenly, granulate, then dry the granulated granules under reduced pressure at 30-40°C for 30 minutes, remove acetone, then add lubricant and mix well, Tablets were compressed according to the required specifications of the drug, coated with Opadry, and the weight was increased by 1%.
Embodiment 3
[0069] A kind of sacubitril valsartan sodium pharmaceutical composition and preparation method thereof, comprising:
[0070]
[0071] (1) Add sacubitril-valsartan sodium and a small amount of acetone to make wet particles over 20 mesh, LCZ696:acetone=1:0.15g / mL;
[0072] (2) Pass the hydrophilic filler-microcrystalline cellulose, the binder-hypromellose, and the disintegrant-croscarmellose sodium through a 80-mesh sieve, and add a small amount of acetone to make a 20-mesh sieve The sum of the mass of wet particles, hydrophilic filler, binder and disintegrant: acetone = 1: 0.5g / mL;
[0073] (3) Mix the wet granules of steps (1) and (2) evenly, granulate, then dry the granulated granules under reduced pressure at 30-40°C for 30 minutes, remove acetone, then add lubricant and mix well, Tablets were compressed according to the required specifications of the drug, coated with Opadry, and the weight was increased by 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com