Oligopeptide ATR001 and monoclonal antibody prepared through oligopeptide and having bias AT1R regulating function and application
A monoclonal antibody and short peptide technology, applied in the direction of antibodies, biochemical equipment and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 hybridoma cell CQ8-A2D9
[0071] 1. Obtaining mouse splenocytes
[0072] 1) Immunization antigen acquisition
[0073] A short peptide ATR001 with a purity of ≥85% was synthesized for the sequence AFHYESQ, and then the short peptide ATR001 was coupled with the carrier protein KLH as an immune antigen;
[0074] 2) Animal immunity
[0075] Immunize 4-6 6-week-old Balb / c mice (live mice) with immune antigens, regular cycle: 3 weeks-2 weeks-2 weeks... intensive cycle: 2 weeks-2 weeks-1 week...use Mouse antiserum ELISA titer ≥ 1:80000, the specific steps are as follows:
[0076] (1) For the first immunization, the amount of immune antigen used: 50ug / monkey, plus Freund's complete adjuvant subcutaneous multi-point injection, with an interval of 3 weeks;
[0077] (2) The second immunization on the 21st day, the dosage route is the same as above, plus Freund's incomplete adjuvant, and the interval is 2 weeks;
[0078] (3) The third immunizati...
Embodiment 2
[0115] 1. Hybridoma subcloning and strain establishment
[0116] ① Preparation of mouse splenocytes as feeder cells;
[0117] ② Prepare the hybridoma cell suspension to be cloned and dilute it with HT medium containing 20% serum to 3 different dilutions containing 5, 10 and 20 cells per ml;
[0118] ③ Add 5×10 per ml 4 -1×10 5 The ratio of cells, respectively add peritoneal macrophages to the above-mentioned hybridoma cell suspension;
[0119] ④ Pack each type of hybridoma into a 96-well plate, with a volume of 100ul per well;
[0120] ⑤37℃, 5% CO 2 After 6 days of culture, the antibody can be detected when there are clones visible to the naked eye; observe under an inverted microscope, mark the wells where only a single clone grows, and take the supernatant for antibody detection.
[0121] ⑥The cells in the wells with positive antibody detection were taken for expansion, cultured, frozen, and subcloned 2-3 times to obtain 2-5 monoclonal cell lines. Hybridoma CQ8-A2D9 ...
Embodiment 3
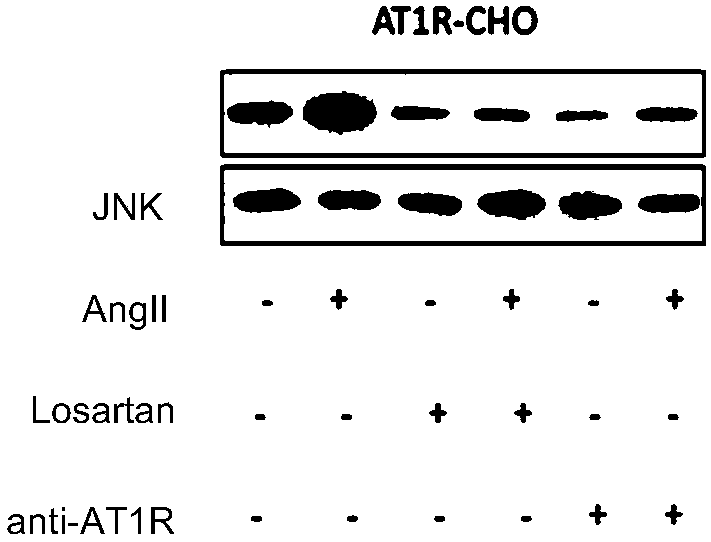
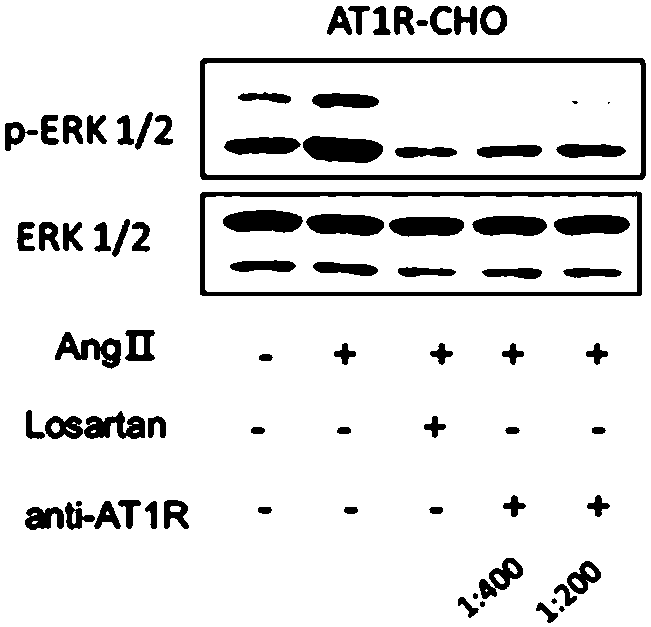
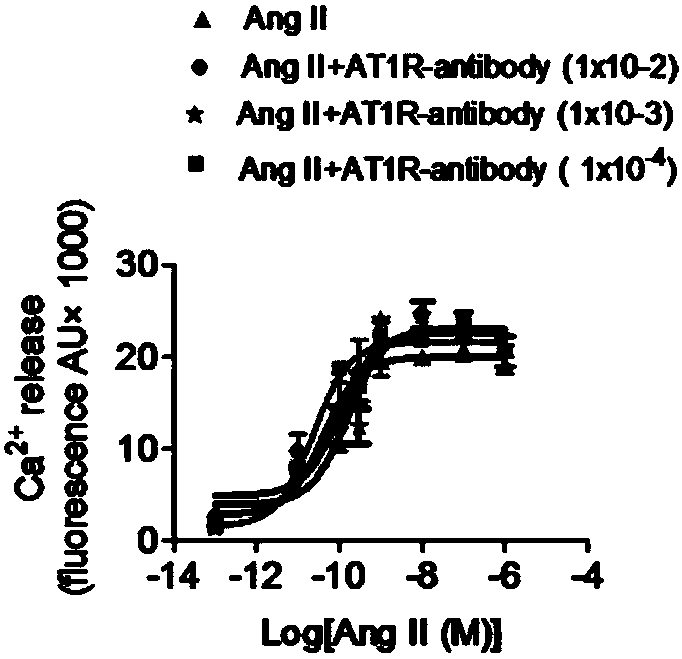
[0132] Example 3 Effect of monoclonal antibody anti-AT1R on phosphorylation of ERK and JNK in CHO cells stably expressing AT1R
[0133] 1. Culture AT1R-CHOK1 cell line (PerkinElmer): DMEM / F12 complete medium containing 10% FBS, 5% CO 2 Cell culture incubator;
[0134] 2. Detection of AngⅡ-induced phosphorylation of ERK and JNK in AT1R-CHOK1
[0135] Inoculate AT1R-CHOK1 in a six-well plate with a density of about 80%, and culture overnight. Then they were starved with HBS for 2h, stimulated by AngⅡconcentration gradient and time gradient, and detected the expression of p-ERK / ERK and p-JNK / JNK by Western Blot.
[0136] 3. Detect the effect of monoclonal antibody anti-AT1R on the phosphorylation of ERK / JNK caused by AT1R activation
[0137] See steps 1 and 2 for cell culture and seeding. After HBS starvation for 2 hours, ARB group and monoclonal antibody group were pre-incubated with losartan and monoclonal antibody anti-AT1R for 1 hour, and then all groups (except the contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com