Shape controllable synthesis method of cuprous oxide powder and application
A technology of cuprous oxide and synthesis method, which is applied in chemical instruments and methods, copper oxide/copper hydroxide, oxidized water/sewage treatment, etc., to achieve the effect of excellent visible light catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Option 1: Add 0.005mol of CuSO 4 ·5H 2 O and 0.005mol of tartaric acid were mixed and added to 80mL of deionized water, and after dissolving, 0.5g of NaOH was added to the above solution to obtain a dark blue solution. Then, the dark blue solution was transferred into a reaction kettle and reacted in an oven at 120° C. for 3 h.
[0023] Scheme 2: Add 0.005mol of CuSO 4 ·5H 2 O and 0.005mol of tartaric acid were mixed and added to 80mL of deionized water, and after dissolving, 0.5g of NaOH was added to the above solution to obtain a dark blue solution. Next, 1.5 g of gelatin was added to the reaction system, heated and dissolved to obtain a dark blue colloidal solution. Finally, the dark blue colloidal solution was transferred into a reaction kettle and reacted in an oven at 120° C. for 3 h.
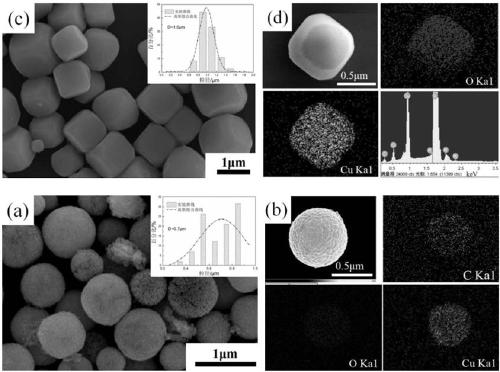
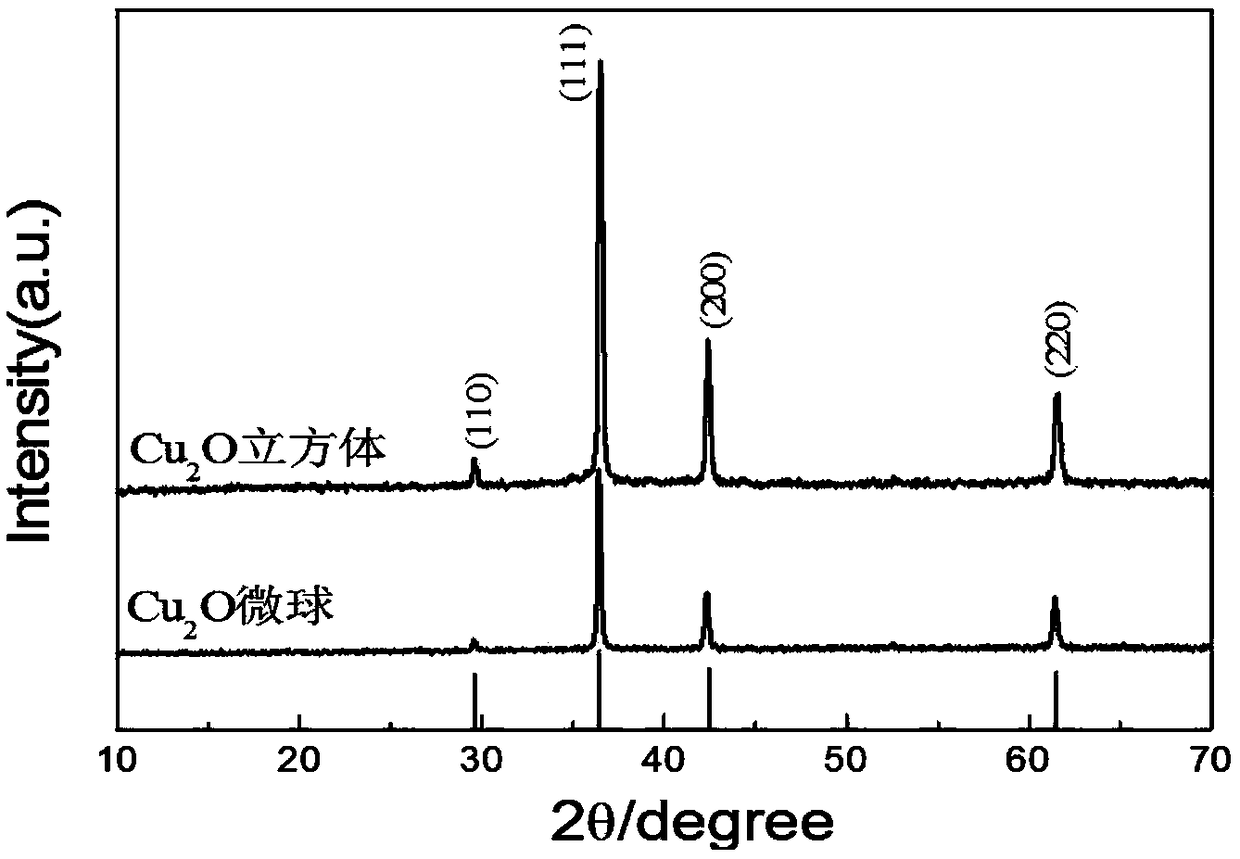
[0024] The morphological characterization of the product prepared in embodiment 1:
[0025] 1. SEM analysis
[0026] It is well known that the morphology of powder particles...
Embodiment 2
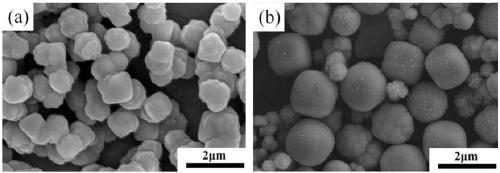
[0030] In order to investigate the effect of tartaric acid usage on Cu 2 The influence of O powder form, preparation condition is the same as embodiment 1, and difference only is that the addition amount of tartaric acid is adjusted to 0.002mol, 0.02mol respectively, and the prepared product is carried out morphological observation, and its result is as follows image 3 shown. The systematic research shows that the amount of tartaric acid in the reaction system has a great influence on the purity and form of the product. When the amount of tartaric acid used is small (0.002mol), there is a small amount of Cu in the product 2 O phase appears ( image 3 shown in a). This is easy to understand, because tartaric acid is a reducing agent, its amount is small, and the reduction reaction is not complete. And when the amount of tartaric acid used was too high (0.02mol), the resulting Cu 2 O is irregular ( image 3 shown in b). Therefore, when the amount of tartaric acid used is...
Embodiment 3
[0032] In order to investigate the effect of the amount of gelatin template usage on Cu 2 The influence of O powder form, preparation condition is the same as embodiment 1, and difference only is that the addition amount of gelatin is adjusted to 0.5g, 2g respectively, then the prepared product is carried out morphological observation, and its result is as follows Figure 4 shown. A series of experimental studies also concluded that the amount of gelatin used mainly affects the shape of the product. When other conditions are the same, spherical Cu 2 O product, when using less (0.5g) (see Figure 4 a), obtained Cu 2 O sphericity is low, and when using too high (2g), there will be gelatin residue in the product (see Figure 4 b). Therefore, the amount of gelatin used for template assembly must also be controlled within a certain range, preferably 1.5 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com