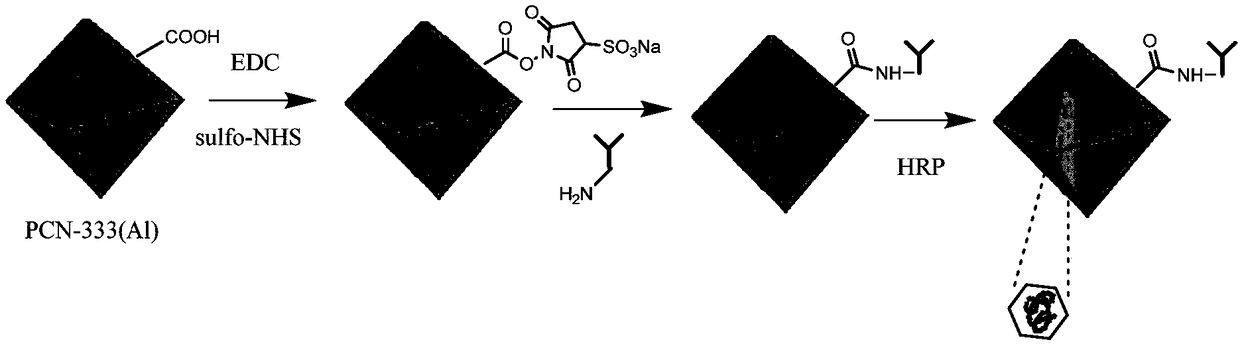
Method for preparing enzyme labeled antibody by utilizing metal organic framework material
A metal-organic framework, enzyme-labeled antibody technology, applied in the field of immunoassays, can solve the problems of low protein loading, insufficient enzyme action, protein variability, etc. Lost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] according to figure 1 The synthetic route shown prepares the enzyme-labeled antibody complex, and the specific method is as follows:
[0031] 1. Synthesis of PCN-333(Al)
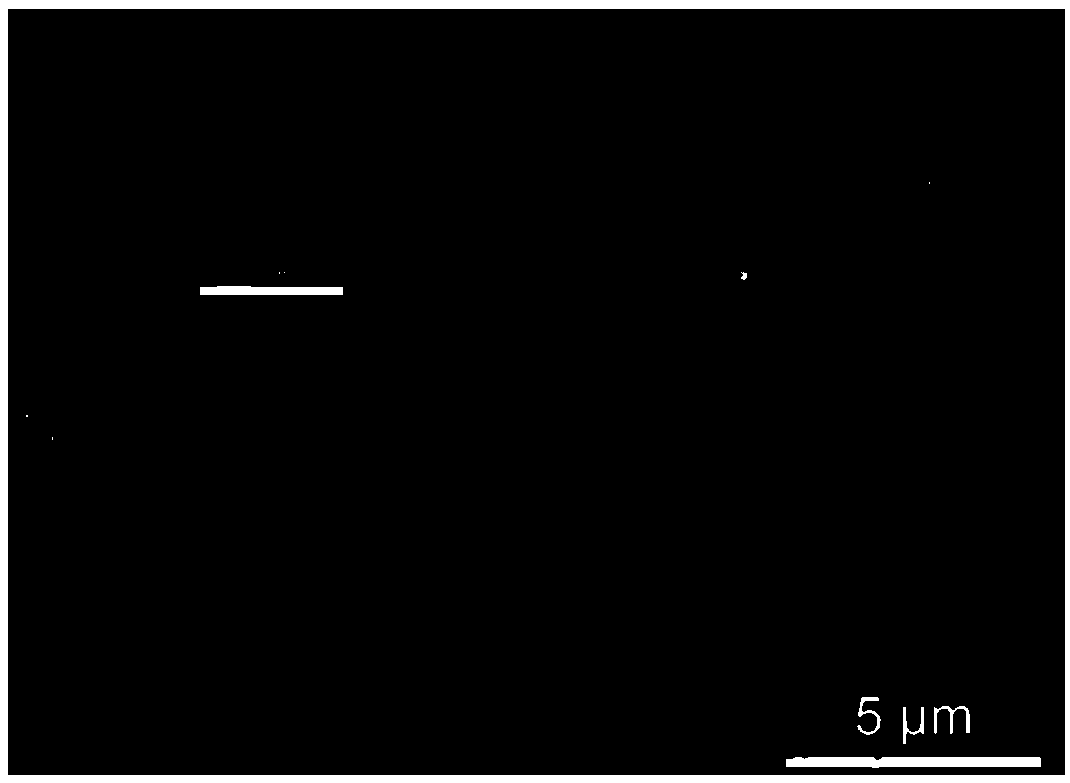
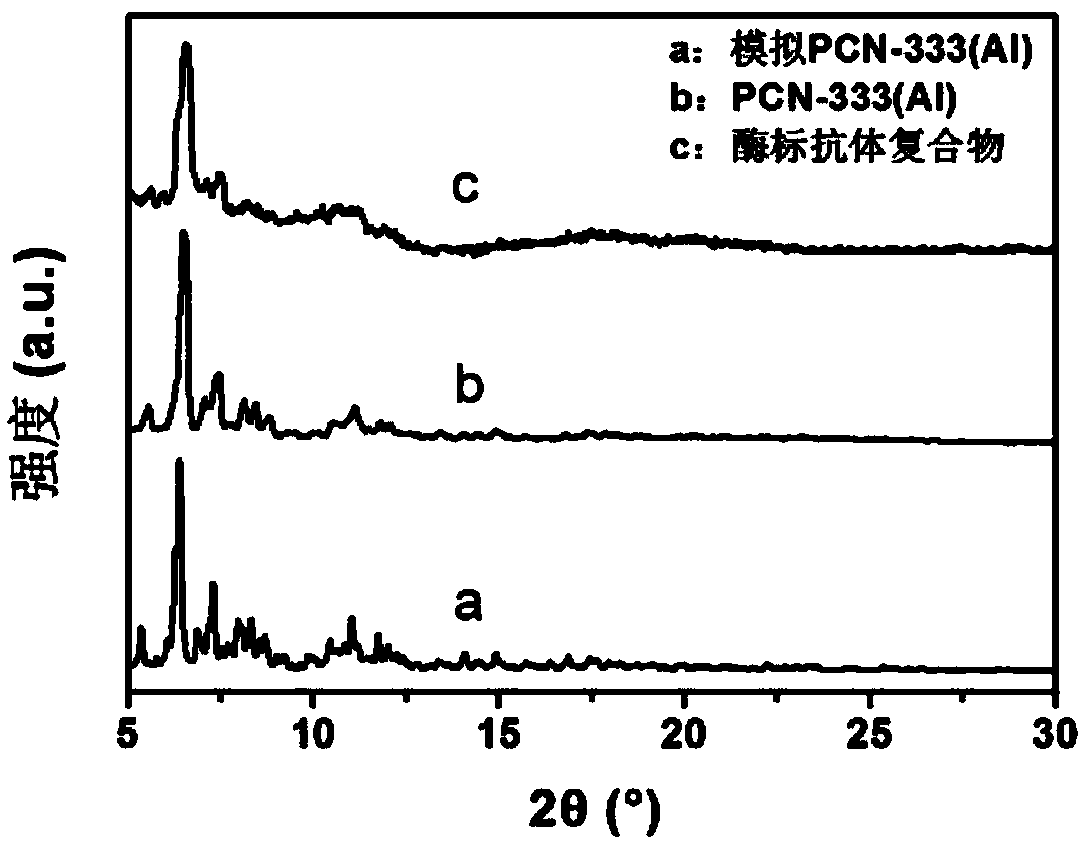
[0032] Take 25mg 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine and 50mg AlCl 3 ·6H 2O. Put 20mg of PVP (average molecular weight 40,000) in a 50mL round bottom flask, add 20mL of N,N-diethylformamide, ultrasonically disperse for 10min, ensure that the added drug is completely dissolved, then add 0.25mL of trifluoroacetic acid, at 120°C Heat and stir in an oil bath for 20 hours, centrifuge, and wash the solid with N,N-dimethylformamide and ethanol to obtain PCN-333(Al). Depend on figure 2 and image 3 It can be seen that 300nm PCN-333(Al) has been successfully synthesized, and the synthesized PCN-333(Al) has uniform size and uniform distribution.
[0033] 2. Functionalized PCN-333(Al)
[0034] Add 1mg PCN-333(Al) to 100μL ultrapure water, then add 100μL 25mmol / L N-(3-dimethylaminopropyl)-N'-ethylc...
Embodiment 2
[0039] In Step 1 of this example, 12.5 mg of 2,4,6-tri(4-carboxyphenyl)-1,3,5-triazine)) and 25 mg of AlCl 3· 6H 2 O. Put 50mg of PVP (average molecular weight 10000) in a 25mL round bottom flask, add 10mL of N,N-diethylformamide, ultrasonically disperse for 10min to ensure that the added drug is completely dissolved, then add 0.125mL of trifluoroacetic acid, and incubate at 120°C Heat and stir in an oil bath for 20 hours, centrifuge, and wash the solid with N,N-dimethylformamide and ethanol to obtain PCN-333(Al). The other steps were the same as in Example 1 to obtain the enzyme-labeled antibody complex, which was stored at 4°C.
Embodiment 3
[0041] In step 1 of this embodiment, take 25mg 2,4,6-tri(4-carboxyphenyl)-1,3,5-triazine)) and 30mgFeCl 3 , 50mg PVP (average molecular weight 10000) in a 25mL round bottom flask, add 10mL N,N-diethylformamide, ultrasonically disperse for 10min to ensure that the added drug is completely dissolved, then add 0.25mL trifluoroacetic acid, oil at 120°C Heated and stirred in the bath for 20 hours, centrifuged, and the solid was washed with N,N-dimethylformamide and ethanol to obtain PCN-333(Fe). The other steps were the same as in Example 1 to obtain the enzyme-labeled antibody complex, which was stored at 4°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com