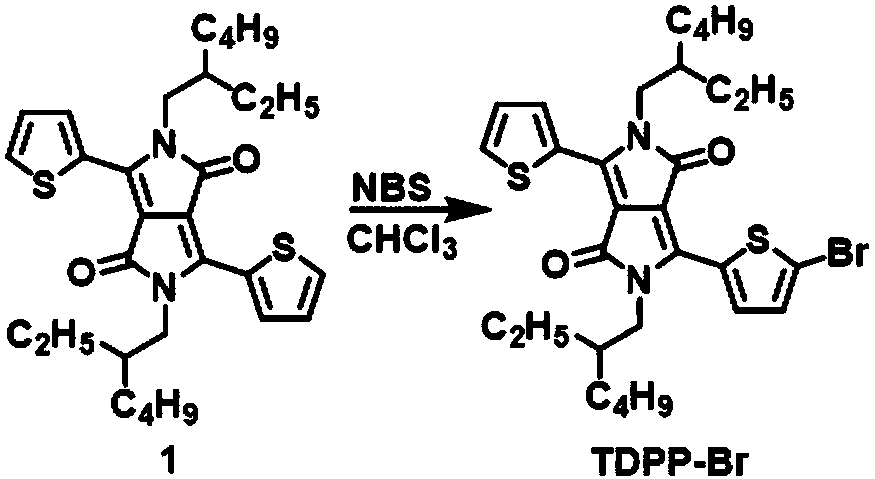
Preparation method of monobromothiophenyl derivatives
A thienyl and derivative technology, applied in the field of chemistry, can solve the problems of high consumption time and cost, low yield of mono-bromothienyl derivatives, difficulty in separation and purification, etc., to simplify subsequent purification and post-treatment processes, avoid The effect of separating and purifying the form and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 100mL of chloroform solution with a concentration of 5mmol / L compound TDPP into a 250mL round bottom flask, keep the reaction system at -20°C, add 10mmol / L N-bromosuccinimide at a rate of 1.0mL / min 50mL of trichloromethane solution, reacted for 8 hours after the addition, and separated the solvent. The crude product was purified by column chromatography with n-hexane and dichloromethane solution with a volume ratio of 5:1 to obtain the isolated product TDPP- Br, the yield was 84%, and the disubstituted TDPP content was zero.
Embodiment 2
[0025] In a 250mL round bottom flask, add 100mL of a chloroform solution with a concentration of 10mmol / L compound TDPP, keep the reaction system at -20°C, and add 10mmol / L of N-bromosuccinimide at a rate of 1.0mL / min 100mL of trichloromethane solution, reacted for 8 hours after the addition, and separated the solvent. The crude product was purified by column chromatography with n-hexane and dichloromethane solution with a volume ratio of 5:1 to obtain the isolated product TDPP- Br, the yield is 95%, and the disubstituted TDPP content is zero.
Embodiment 3
[0027] Add 50mL of chloroform solution with a concentration of 20mmol / L compound TDPP into a 250mL round bottom flask, keep the reaction system at -20°C, add 10mmol / L N-bromosuccinimide at a rate of 1.0mL / min 100mL of trichloromethane solution, reacted for 8 hours after the addition, and separated the solvent. The crude product was purified by column chromatography with n-hexane and dichloromethane solution with a volume ratio of 5:1 to obtain the isolated product TDPP- Br, the yield was 86%, and the disubstituted TDPP content was zero.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com