A kind of preparation method of 2,4-dichloropyrimidine and its derivatives
A technology of dichloropyrimidine and its derivatives, which is applied in the field of preparation of 2,4-dichloropyrimidine and its derivatives, can solve the problems of large phosphorus-containing wastewater discharge and unfriendly environment, and achieve large discharge and reduce environmental pollution Problems, the effect of high conversion rate of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
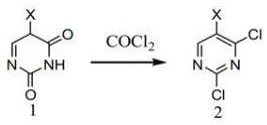
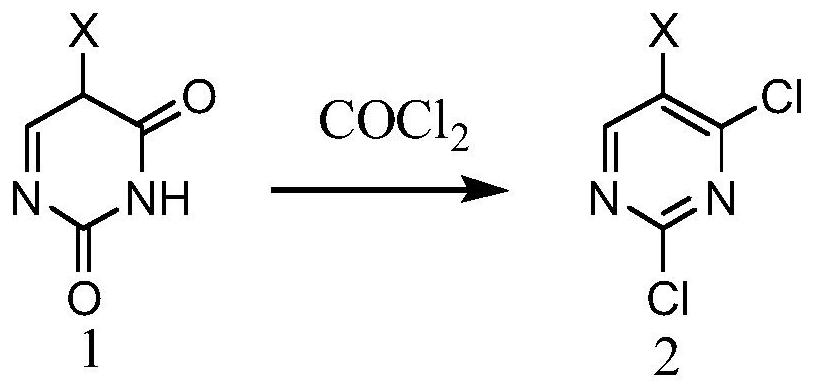
[0025]A method for preparing 2,4-dichloropyrimidine and its derivatives, comprising a catalyst, compound 1 and phosgene, in the solvent, the compound 1 is reacted with phosgene under the action of the catalyst;
[0026]The reaction of the compound 1 on the catalyst is as follows:
[0027]
[0028]X is any one of H, Cl, Br;
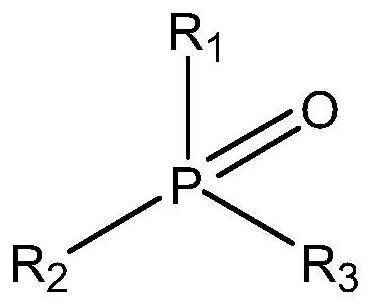
[0029]The structure of the catalyst is as follows:
[0030]
[0031]R1 is C1-10, R2=C1-10, R3=C1-10, R1, R2, R3 are the same, preferably R3PO compounds, where R=C1-10 alkyl or phenyl, preferably triphenylphosphine oxide , Tributyl phosphine oxide and trioctyl phosphine oxide, the catalyst can be reused.
[0032]Which includes the following steps:
[0033]Step 1: Take compound 1, catalyst and solvent into a three-necked flask with a thermometer, agitator, gas tube, and condenser (-5 to -10°C) to prepare the mixture, and use nitrogen to check for leaks;
[0034]Step 2: Heat the mixture prepared in step 1 to 20°C to 90°C, and pass the phosgene under the reaction liquid surface through the ga...
Embodiment 1
[0044]Uracil (51.8 g, 98% content; 0.45 mol), triphenylphosphine oxide (31.3 g, 98% content; 0.11 mol), and nitrobenzene (100 ml) are packed in 250 ml with a thermometer In a three-necked flask with stirring, gas pipe and condenser (-5—-10℃), the system uses nitrogen to check for leaks. The uracil mixture was heated to 60°C, and phosgene (180 g, 99% content; 1.8 mol) was passed through the airway to the reaction liquid surface. During the addition, a small amount of phosgene began to reflux. The phosgene penetration time is 20 minutes. The mixture was heated to 105°C. When the temperature is 90°C, a large amount of gas is released, and a large amount of phosgene in the condenser is refluxed. After 20 minutes, the reaction mixture was clear red. The gassing rate after 40 minutes was very slow; the reaction solution was sampled and the reaction was continued for 1 hour, during which all gassing had ceased. HPLC analysis indicated that the reaction was complete. The reaction mixture wa...
Embodiment 2
[0047]5-Bromouracil (191 g, 99.5% content; 1 mol), tributyl phosphine oxide (111.2 g, 98% content; 0.5 mol) and butyronitrile (200 ml) are packed into 1000 ml tape In a three-necked flask with thermometer, stirring, constant pressure funnel, and condenser (-5 to -10°C), the system uses nitrogen to check for leaks. The 5-bromouracil mixture was heated to 80°C, and solid phosgene (320 g, 99% content; that is, 3.2 moles) and 400 ml of butyronitrile solution were added dropwise to the reaction solution via a constant pressure funnel. During the dropping process, a small amount of phosgene began to reflux. The phosgene addition time is 70 minutes. The mixture was heated to 125°C. When the temperature is 90°C, a large amount of gas is released, and a large amount of phosgene in the condenser is refluxed. After 40 minutes, the reaction mixture was clear red. The gassing rate after 40 minutes was very slow; the reaction solution was sampled and the reaction was continued for 1 hour, during ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com