MG53 protein/MG53 mutant protein enteric capsule and preparation method thereof
An enteric-coated capsule and mutant technology, applied in the medical field, can solve the problems of being easily degraded and the active ingredients of drugs cannot be guaranteed, and achieve the effects of shortening the medication cycle, alleviating pain, and increasing the distribution area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
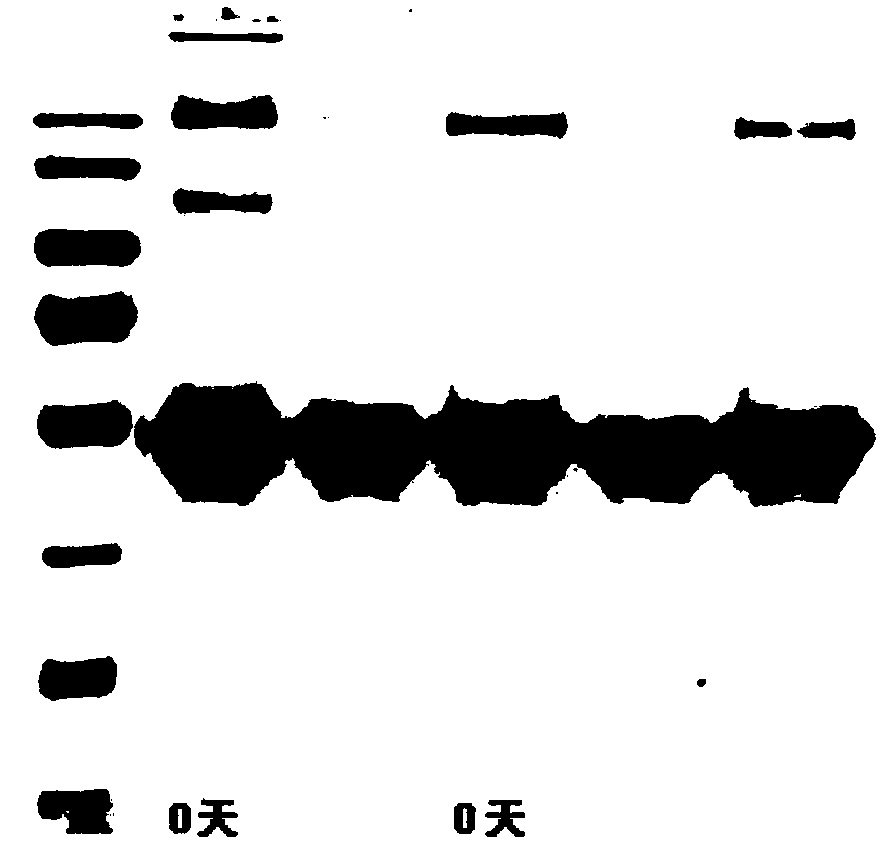
Image
Examples
Embodiment 1
[0038] A MG53 protein / MG53 mutant protein enteric-coated capsule, including MG53 protein / MG53 mutant protein enteric-coated pellets and a blank enteric-coated capsule;
[0039] Configuration of MG53 protein / MG53 mutant protein enteric-coated pellets:
[0040]
[0041] making process:
[0042] S1. Weigh the pellet core, place it in a fluidized bed to preheat, the temperature is 40-55°C, and the time is 15-45min;
[0043] S2. Preparation of MG53 protein / MG53 mutant protein enteric-coated pellets: Weigh, take the lyophilized powder of MG53 or its mutant, add it to water and stir; add the binder to the lyophilized powder solution of MG53 or its mutant Stir to disperse evenly,
[0044] Adjust the parameters and apply the above-mentioned solution. The specific parameters are as follows:
[0045] Inlet speed 18~22m 3 / h·100g, air inlet temperature 43℃~48℃, material temperature 35℃~38℃, fan speed 1700~1900rpm, atomization pressure 0.2~0.23MPa, nozzle diameter 0.3mm.
[0046] ...
Embodiment 2
[0055] The difference between the present embodiment and the above-mentioned implementation is that the binder is: povidone (PVP), hypromellose (HPMC), carmellose sodium (CMC-Na), syrup; preferably , the present embodiment selects hypromellose, and the hypromellose has thickening ability, salt tolerance, low ash powder, pH stability, water retention, dimensional stability, excellent film-forming property, and extensive Enzyme resistance, dispersibility and cohesiveness and other characteristics.
Embodiment 3
[0057] The difference between this embodiment and the above-mentioned embodiments is that the pellet core is a microcrystalline cellulose pellet core, a sucrose pellet core, a starch pellet core, and the like. Preferably, the microcrystalline cellulose pellet core is selected in this implementation. The particle diameter of the pellet core is 100-1000 μm. Compared with sugar spherical particles, it has moderate water absorption, and the adhesion between particles is smaller, so it can be more easily coated with drugs and can effectively improve production efficiency.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com