Preparation and application of mitochondrial-targeting self-assembled protein nanoparticles
A nanoparticle and protein technology, applied in peptide/protein components, preparations for in vivo tests, metallothionein, etc., can solve the problems of short half-life, limited application, and ineffective enrichment of small molecules, and achieve biocompatibility Good sex and water solubility, reduced damage, fast targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
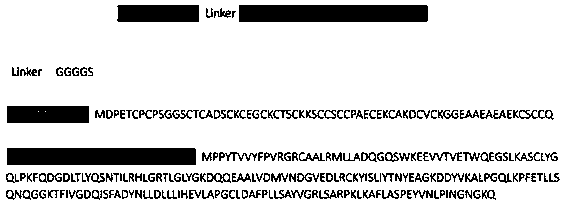
[0055] Embodiment 1, construct the protein nanoparticle amino acid sequence of GSTP1-MT3 mitochondria targeting:
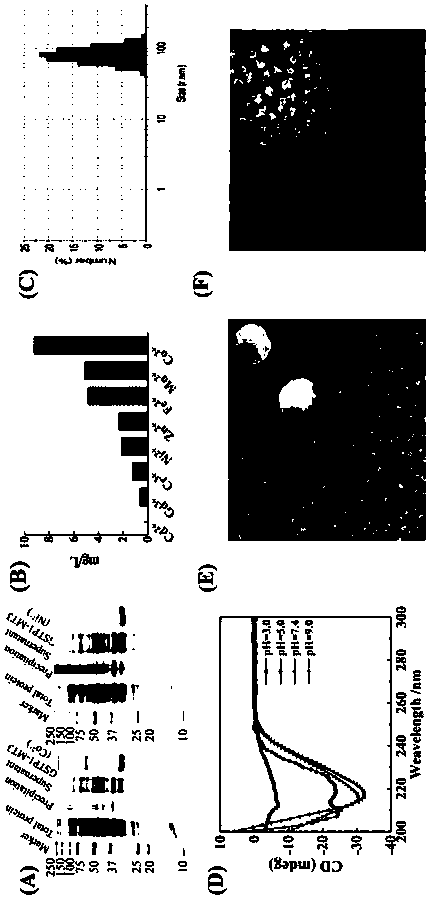
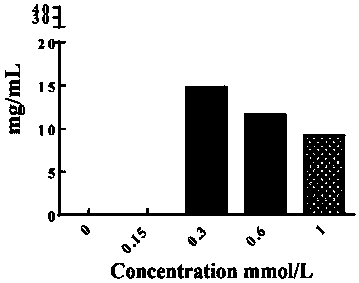
[0056] Based on previous experimental studies, the following mitochondrial-targeted protein nanoparticle expression vectors were constructed, such as figure 1 As shown, GSTP1-MT3 is mainly composed of GSTP1 and MT3, wherein MT3 is at the N-terminus of the amino acid sequence, GSTP1 is at the C-terminus of the amino acid sequence, and MT3 and GSTP1 are coupled through the GGGGS sequence.
[0057] GSTP1 amino acid sequence:
[0058] MPPYTVVYFPVRGRCAALRMLLADQGQSWKEEVVTVETWQEGSLKASCLYGQLPKFQ DGDLTLYQSNTILRHLGRTLGLYGKDQQEAALVDMVNDGVEDLRCKYISLIYTNYEAGKDDYVK ALPGQLKPFETLLSQNQGGKTFIVGDQISFADYNLLDLLLIHEVLAPGCLDAFPLLSAYVGRLSAR PKLKAFLASPEYVNLPINGNGKQ(SEQ IDNO:1);
[0059] MT3 amino acid sequence:
[0060] MDPETCPCPSGGSCTCADSCKCEGCKCTSCKKSCCSCCPAECEKCAKDCVCKGGEAAEAEAEKCSCCQ (SEQ ID NO: 2);
[0061] Linker between GSTP1 and MT3:
[0062] GGGGS (SEQ ID NO: 3).
[0063] Th...
Embodiment 2
[0064] Example 2. Construction of GSTP1-MT3 protein nanoparticle carrier
[0065] In order to facilitate the subsequent expression of GSTP1-MT3 protein nanoparticles in recombinant cells, its prokaryotic expression vector was constructed, wherein pET-28a(+) was selected as the prokaryotic expression vector, and the restriction sites HindIII and NdeI on it were used to convert The nucleotide sequence encoding GSTP1-MT3 shown in SEQ ID NO: 5 was ligated into pET-28a(+), and the recombinant expression vector pET-28a(+)-GSTP1 was successfully obtained after enzyme digestion, electrophoresis and monoclonal sequencing detection -MT3.
Embodiment 3
[0066] Example 3. Recombinant expression of GSTP1-MT3 protein nanoparticles
[0067] Using Escherichia coli as the host bacteria for recombinant expression, the specific expression method is as follows:
[0068] 1. Plasmid transformation
[0069] Take 2 μL 42ng / μL pET-28a(+)-GSTP1-MT3 plasmid, add it to 20 μL BL21(DE3) competent cells, pre-mix on ice for 15-30 minutes, then place in a 42°C water bath and heat for 90 seconds. Then keep on ice for another 10 minutes. Add 800 μL of non-resistant LB medium, incubate at 37°C 220 rpm for 1 hour, then centrifuge at 3500 rpm for 10 minutes, remove 600 μL of supernatant, and mix the remaining 200 μL for use.
[0070] 2. Resistance screening
[0071] Add the remaining 200 μL of the bacterial solution in step 1 to the agarose plate containing kanamycin, incubate in a 37°C incubator for 2 hours, and incubate the plate upside down overnight.
[0072] 3. Monoclonal selection
[0073] A single clone was selected, added to 10 mL of LB me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Isoelectric point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com