Method for preparing hindered phenolic antioxidant by using composite catalyst
A composite catalyst, technology of hindered phenols, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve elimination, human and environmental hazards, and low reaction rate. and other problems to achieve the effect of reducing the reaction time, ensuring product quality and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
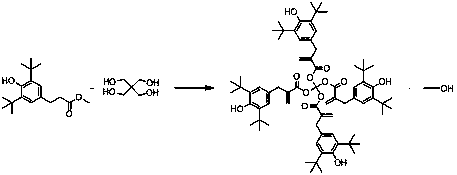
[0030] (1) In a reactor equipped with a stirrer, condenser, vacuum gauge, and thermometer, put 145kg (1.07kmol) of pentaerythritol, 1620kg (5.54kmol, excess 30mol%) of 35 methyl ester melted at 80°C, and evenly After mixing, add 0.49kg (0.021kmol) of lithium amide and 0.35kg (0.0053kmol) of lithium acetate under nitrogen protection; when the mixture is heated to 130-135°C at 10,000pa, the internal pressure of the reaction begins to change, about 11,000pa, It shows that the materials in the kettle start to react, and when the temperature in the kettle reaches 140°C, a large number of bubbles are generated on the surface of the kettle, and the pressure of the kettle also changes to about 13000-15000pa, indicating that the reaction in the kettle is violent, and the temperature of the kettle must be controlled at about 140℃, otherwise it will A large amount of reactant is taken out of the kettle by the by-product methanol. When the kettle temperature reaches 140°C, keep it at this...
Embodiment 2
[0038] Reaction process is with embodiment 1, and the pentaerythritol of 145kg (1.07kmol) is loaded into reactor, the 35 methyl esters of 1995kg (6.82kmol, excessive 60mol%), the amide lithium of 0.61kg (0.027kmol) and 0.35kg (0.0053kmol) lithium acetate. After the distillation of 3,5-methyl ester, about 4-6 hours later, the degree of vacuum is about 100 Pa, the temperature is 195-200°C, and the value of distilled methyl ester is 732kg.
[0039] Yield: 1204kg (96.0% of theoretical amount) of crystal 1010, melting point 118°C-119°C. Product light transmittance: 425nm: 98.2%, 500nm: 99.4%.
Embodiment 3
[0041] Reaction process and other materials add-on are the same as embodiment 1, just, 35 methyl esters 1370kg (4.69kmol, excessive 10mol%). After the distillation of 3,5-methyl ester, about 2-3 hours later, the degree of vacuum is about 100 Pa, the temperature is 195-200°C, and the value of distilled methyl ester is 116kg.
[0042] Yield: 1141kg (91.0% of theoretical amount) of crystal 1010, melting point 116°C-118°C. Product light transmittance: 425nm: 97.7%, 500nm: 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com