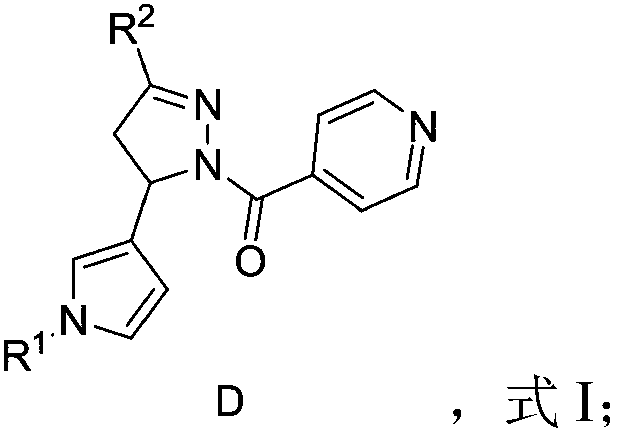
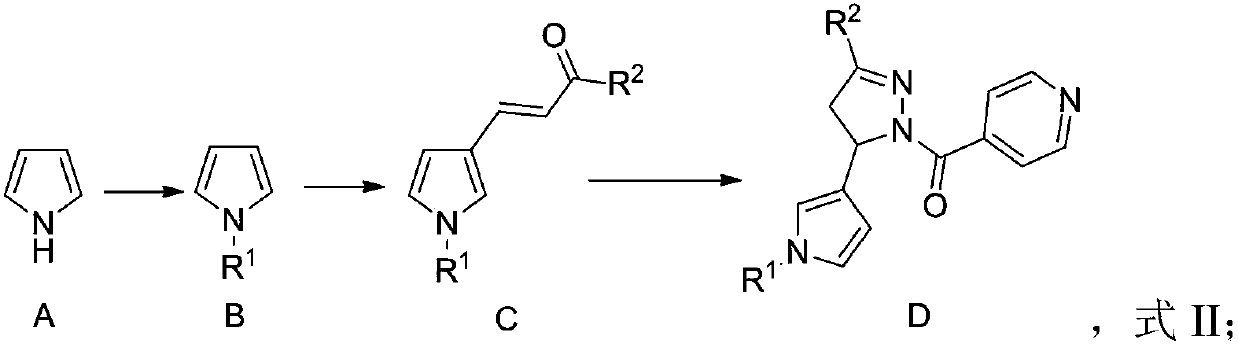
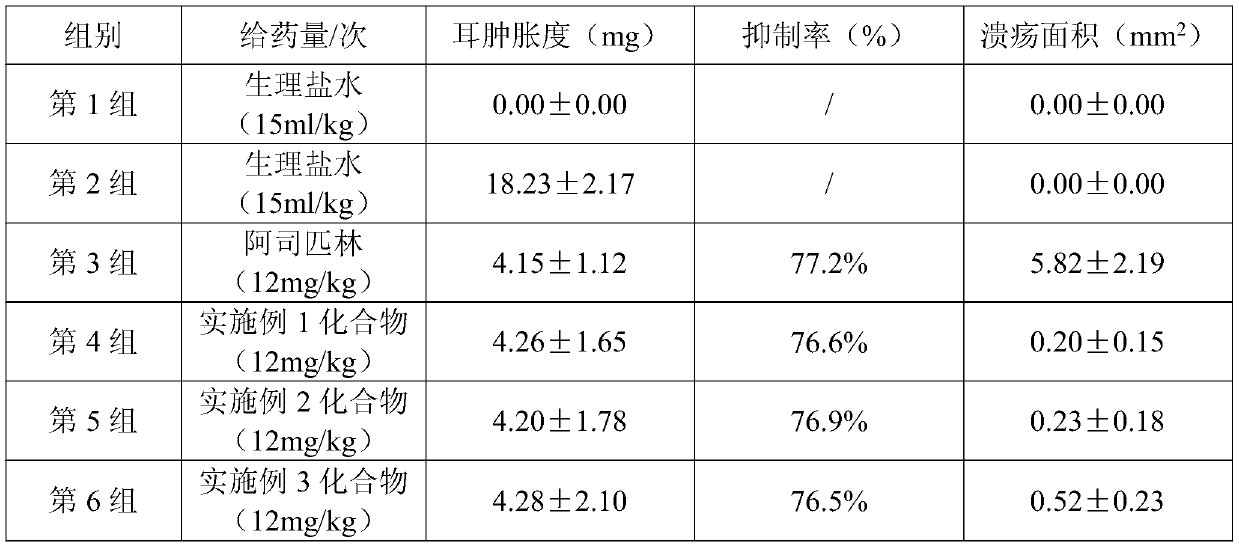
Anti-inflammatory COX/LOX inhibitor, and preparation method and application thereof
A technology of inhibitor and synthesis method, which is applied in the directions of anti-inflammatory agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of poor selectivity of NSAIDs, limited wide application, intestinal toxicity and side effects, etc., and achieves a simple preparation method. Easy to operate, reduce ear swelling, less gastrointestinal irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 preparation
[0039] (1) The synthesis method is as follows:
[0040] Take pyrrole (10mmol), CH 2 CH 3 OCH 2Add Br (11mmol) and sodium hydride (12mmol) to 20mL dimethylformamide, stir at 40°C for 5 hours to obtain a reaction solution; add saturated aqueous sodium chloride solution and ethyl acetate to extract the reaction solution , the organic phase was concentrated and purified by column, and the purified solution was collected and distilled under reduced pressure to obtain 1.14 g (9.1 mmol) of 1-(ethoxymethyl)-1H-pyrrole, with a yield of 91%.
[0041] (2) The synthesis method is as follows:
[0042] Take 1-(ethoxymethyl)-1H-pyrrole (5mmol), (6mmol), zinc chloride (4mmol), join in 10mL dichloromethane, stir 6 hours at room temperature, obtain reaction liquid; Add saturated sodium chloride aqueous solution, ethyl acetate extraction in described reaction liquid, organic phase After concentration, the column was purified, and the purified soluti...
Embodiment 2
[0046] Embodiment 2 preparation
[0047] (1) The synthesis method is as follows:
[0048] Take pyrrole (10mmol), methyl iodide (11mmol), sodium hydroxide (11mmol), join in 20mL dimethyl sulfoxide, stir at room temperature for 5 hours, obtain reaction solution; Add saturated sodium chloride to the reaction solution The aqueous solution and ethyl acetate were extracted, the organic phase was concentrated and purified by column, and the purified solution was collected and distilled under reduced pressure to obtain 753 mg (9.3 mmol) of N-methylpyrrole, with a yield of 93%.
[0049] (2) The synthesis method is as follows:
[0050] Take N-methylpyrrole (5mmol), (6mmol), aluminum chloride (4mmol), join in 10mL methylene chloride, stir 4 hours at room temperature, obtain reaction solution; Add saturated sodium chloride aqueous solution, ethyl acetate extraction in described reaction solution, organic After the phase was concentrated, the column was purified, and the purified...
Embodiment 3
[0054] Example 3 Preparation
[0055] (1) The synthesis method is as follows:
[0056] Take pyrrole (10mmol), benzyl bromide (12mmol), and potassium carbonate (15mmol), add them to 25mL of tetrahydrofuran, and stir at room temperature for 6 hours to obtain a reaction solution; add saturated aqueous sodium chloride solution, acetic acid After extraction with ethyl ester, the organic phase was concentrated and then purified by column. The collected purified solution was distilled under reduced pressure to obtain 1.49 g (9.5 mmol) of N-benzylpyrrole with a yield of 95%.
[0057] (2) The synthesis method is as follows:
[0058] Take N-benzylpyrrole (5mmol), (6mmol), zinc chloride (4mmol), join in 10mL chloroform, stir 4 hours at room temperature, obtain reaction solution; Add saturated sodium chloride aqueous solution, ethyl acetate extraction in described reaction solution, after the organic phase concentrates After column purification, the purified liquid was collected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com