Synthesis method and application of o-vanillin isonicotinoyl hydrazone iron and nickel complex
A technology of ortho-vanillin and nicotinyl hydrazone nickel ortho, which is applied in the field of bio-inorganic chemistry and achieves the effects of being convenient for industrialized production, readily available and cheap raw materials, and simple and feasible preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
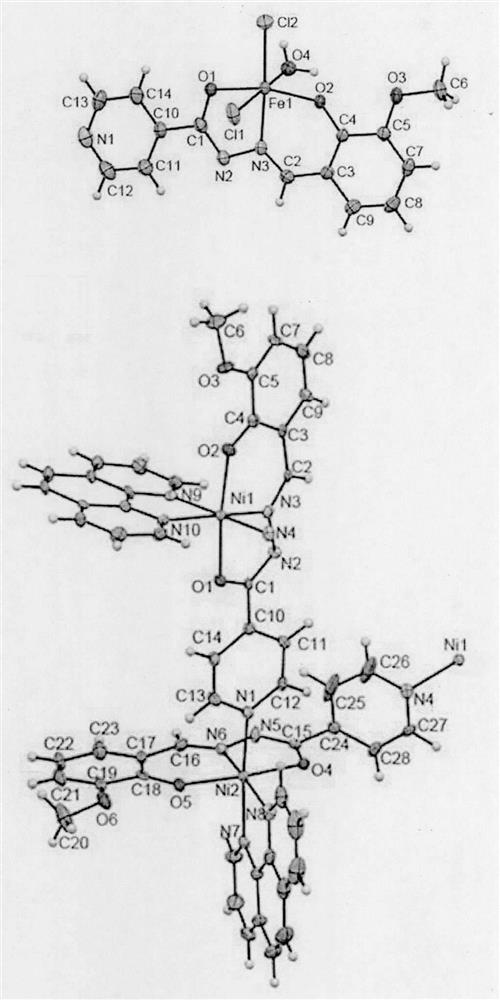
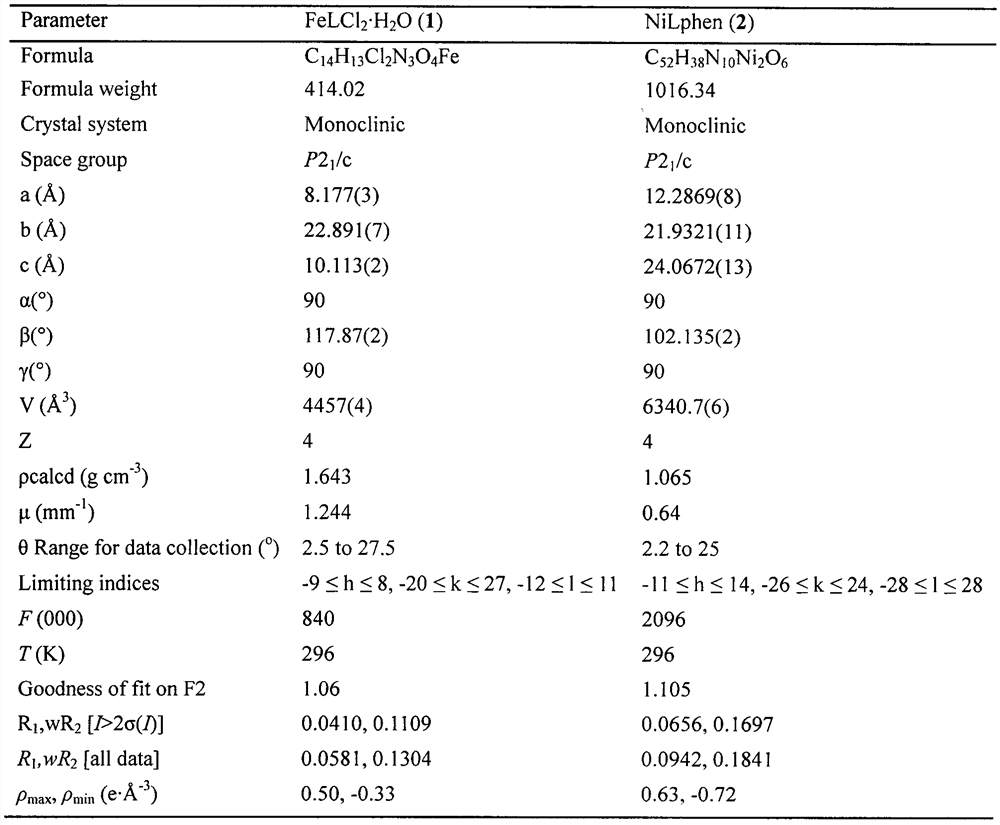
[0020] FeLCl 2 ·H 2 The specific steps of the synthesis method of O are as follows: dissolving o-vanillin isonicotidine hydrazone (27.1 mg, 0.1 mmol) in 10 mL of MeOH, heated and stirred, and FeCl 3 ·6H 2 O (27.0 mg, 0.1 mmol) was dissolved in 3 mL of MeCN and slowly added dropwise to the ligand solution to obtain an amber solution, which was allowed to reflux for 3 h and then cooled to room temperature, and slowly volatilized for two weeks to obtain a black flaky single crystal. Its crystal structure is shown in figure 1 .
Embodiment 2
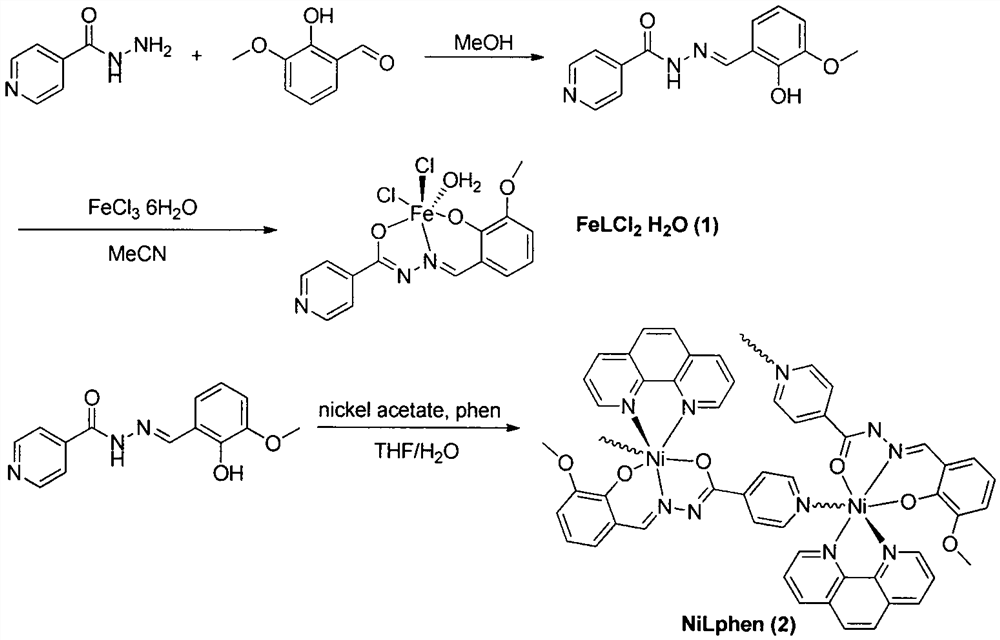
[0022] The specific steps of the synthetic method of NiLphen are: dissolving o-vanillin isonicotidine hydrazone (27.1mg, 0.1mmol) in 12mLTHF / H 2 O mixed solution, and added 30 μL Et 3 N, stirring, Ni(OAc) 2 4H 2 O (24.8mg, 0.1mmol) dissolved in 12mL THF / H 2 The O mixed solution was then added dropwise to the ligand solution, and finally 18 mg of 1,10-phenanthroline was added to obtain an orange-yellow solution, which was sonicated until clear. After filtering, let it stand for slow volatilization for a week to obtain orange flaky single crystal. Its crystal structure is shown in figure 1 .
[0023] In vitro anti-tumor activity research was carried out for the above complexes, and the Cell Counting Kit-8 method was used to measure the inhibitory rates of complexes 1 and 2 on human lung cancer cells (A549) and human cervical cancer cells (HeLa), and the IC50 (μM) was calculated. The results are shown in Table 3.
[0024] The Cell Counting Kit-8 is called for short CCK-8 k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com