Method for detecting pyruvic acid and concentration thereof by fluorescent gold nanocluster probe, method for detecting pyruvate oxidase and concentration thereof
A technology of pyruvate oxidase and fluorescent gold nanometers, which is applied in the direction of material analysis, fluorescence/phosphorescence, and measurement devices by optical means, can solve the problems of expensive equipment, increase the cost of users, and complicated operations, and achieve excellent fluorescence properties. , good selectivity, wide linear range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
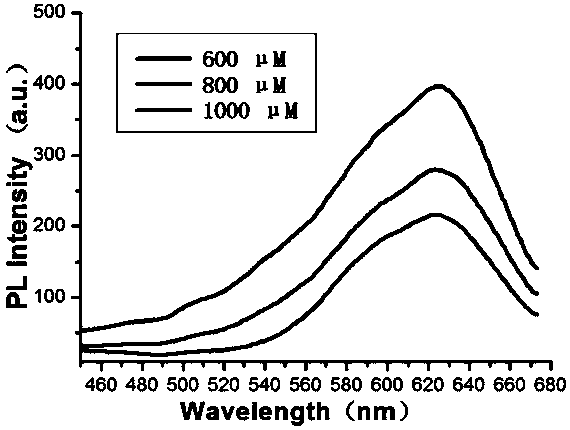
[0161] (1) Take 3700 μL of pyruvate solution (600 μM) and blend it with 250 μL of pyruvate oxidase at a concentration of 1 mg / mL. Then place it in a 43°C water bath and incubate for 2.5 hours;
[0162] (2) Add 350 μL of disodium hydrogen phosphate-citric acid buffer solution (concentration: 1 mM, pH=4.3) and 250 μL of N-2-pyrrolidone (concentration: 40 mM) to the solutions of various concentrations in the above step (1). , Cu 2 SO 4 60μL (concentration is 50mM). Shake well and react at room temperature for 65 minutes. Then dialyze with a dialysis bag with a molecular weight of 1000Da for 18 hours;
[0163] (3) Take 600 μL of the solution prepared in step (2), and add HAuCl in sequence 4 250 μL (100 mM), 75 μL of acetic acid-sodium acetate buffer solution (pH=4.5), and shake to mix evenly. The sample was placed in a microwave oven, the power was set to 600w, and the time was 9 minutes to obtain gold nanoclusters with strong fluorescence;
[0164] (4) Steps (1)-(3) were...
Embodiment 2
[0172] (1) Take 3500 μL of pyruvate solution (100 μM), and blend it with 100 μL of pyruvate oxidase at a concentration of 0.5 mg / mL. Then place it in a 40°C water bath and incubate for 2 hours;
[0173] (2) Add 100 μL of disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution (concentration: 0.5 mM, pH=5.5) and 100 μL of N-2-pyrrolidone (concentration: 20mM), oxalic acid 10μL (30mM concentration). Shake well and react at room temperature for 10 minutes. Then dialyze for 12 hours with a dialysis bag with a molecular weight of 500 Da;
[0174] (3) Take 200 μL of the solution prepared in step (2), and add HAuCl in sequence 4 100 μL (50 mM), 50 μL of disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution (pH=5.2), and shake to mix evenly. Put the sample in a microwave oven with the power set to 400w for 15 minutes to obtain gold nanoclusters with strong fluorescence;
[0175] (4) Steps (1)-(3) were repeated to measure the fluorescence e...
Embodiment 3
[0177] (1) Take 4000 μL of pyruvate solution (1600 μM) and blend it with 500 μL of pyruvate oxidase at a concentration of 2 mg / mL. Then place it in a 45°C water bath and incubate for 3 hours;
[0178] (2) Add 500 μL of glycine-hydrochloric acid buffer solution (concentration: 2 mM, pH=3.2), 400 μL of N-2-pyrrolidone (concentration: 60 mM), NaHSO 3 100μL (concentration is 70mM). Shake well and react at room temperature for 120min. Then dialyze with a dialysis bag with a molecular weight of 1500Da for 24 hours;
[0179] (3) Take 1000 μL of the solution prepared in step (2), and add HAuCl in sequence 4 500 μL (200 mM), 100 μL of citric acid-sodium hydroxide-hydrochloric acid buffer solution (pH=4.2), and shake to mix evenly. Put the sample in a microwave oven with the power set to 800w for 3 minutes to obtain gold nanoclusters with strong fluorescence;
[0180] (4) Steps (1)-(3) were repeated to measure the fluorescence emission spectra of other pyruvate solutions (1800 μM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com