Flue gas denitrification catalyst and its preparation method and application
A denitration catalyst and flue gas technology, applied in the field of denitration catalysts, can solve the problems of poor wear resistance of the coating, poor poisoning ability, easy decline in activity, etc., and achieve the effects of low cost, good thermal stability and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
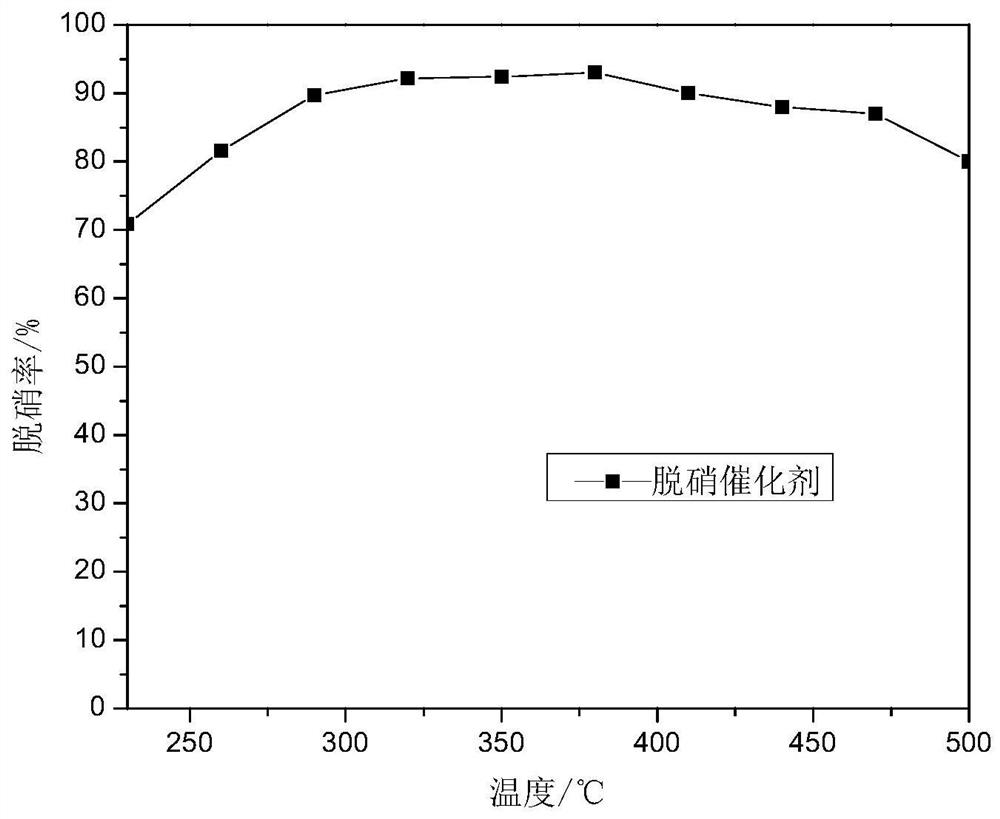
Image
Examples
preparation example Construction
[0037] The present invention also provides a method for preparing a flue gas denitrification catalyst, the method comprising:
[0038] 1) Mix solution A and solution B to obtain solution C, and then contact solution C with urea to obtain solution D;
[0039] 2) The solution D is heated, solid-liquid separated, and first dried to obtain catalyst powder;
[0040] 3) The catalyst powder and the filler are mixed and formed, secondly dried, and roasted to obtain a flue gas denitration catalyst,
[0041] Wherein, the solution A contains titanium salts and zirconium salts, and the solution B contains praseodymium salts, lanthanum salts, erbium salts, and tungsten salts, iron salts, cerium salts, gold salts, silver salts, and rhodium salts. one or more;
[0042] In terms of Ti / Zr elements, the molar ratio of the titanium salt to the zirconium salt is 1: (0.1-0.7);
[0043] In terms of Pr / La / Er elements, the molar ratio of the praseodymium salt, the lanthanum salt and the erbium sal...
Embodiment 1
[0064] (1) Preparation of precursor solution
[0065] With the molar ratio of Ti / Zr as 1:0.2, weigh 153.1g of titanyl sulfate and 61.7g of zirconium oxychloride, dissolve them in 500g of deionized water, and add 50ml of concentrated sulfuric acid with a mass fraction of 98% to the above solution , and stirred at 60 °C until a clear solution was obtained to obtain solution A. Weigh praseodymium nitrate, lanthanum nitrate, erbium nitrate, ammonium metatungstate, ferric chloride, cerium chloride, dissolve it in 500g deionized water, obtain solution B, wherein, the amount of each salt in solution B meets the following ratio, according to Relative to the total amount of titanium salt and zirconium salt contained in solution A in terms of oxides of each metal, the total amount of praseodymium salt, lanthanum salt and erbium salt contained in solution B in terms of oxides of each metal is 20 Mass %, the total amount of tungsten salt, iron salt and cerium salt contained in solution B...
Embodiment 2
[0073] (1) Preparation of precursor solution
[0074] With the molar ratio of Ti / Zr as 1:0.5, weigh 113.2g of titanyl sulfate and 114.0g of zirconium oxychloride, dissolve them in 500g of deionized water, and add 50ml of concentrated sulfuric acid with a mass fraction of 98% to the above solution , and stirred at 60 °C until a clear solution was obtained to obtain solution A. Weigh praseodymium nitrate, lanthanum nitrate, erbium nitrate, and ammonium tungstate, and dissolve them in 500g of deionized water to obtain solution B, wherein the amount of each salt in solution B satisfies the following proportions, calculated according to the oxides of each metal The total amount of the titanium salt and the zirconium salt contained in the solution A, the total amount of the praseodymium salt, the lanthanum salt and the erbium salt contained in the solution B in terms of the oxides of each metal is 25 mass%, and the oxides of each metal The tungsten salt contained in the calculated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com